Regioselective Acylation of Levoglucosan Catalyzed by Candida Antarctica (CaLB) Lipase Immobilized on Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Immobilization Procedures
2.2.1. Immobilization of Lipase CaLB
2.2.2. Hydrolytic Activity Assay
2.2.3. Levoglucosan Derivatization Method
Silylation with BSTFA and MSTFA
Silylation with tert-butyl Dimethyl Silane Chloride
2.3. Lipase-Catalyzed Acylation of Levoglucosan
2.3.1. Esterification of Long-Chain Fatty Acids
2.3.2. Transesterification of Levoglucosan
2.4. Gas Chromatography Analysis
3. Results and Discussion
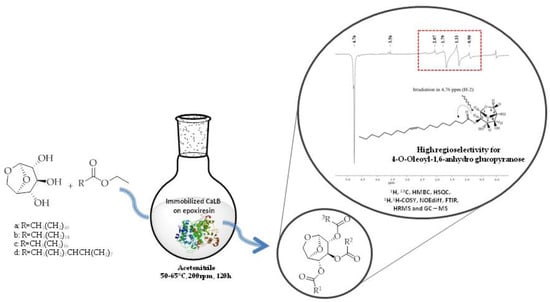
Lipase-catalyzed Acylation of 1,6-anhydroglucopyranose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morais, A.R.; Bogel-Lukasik, R. Green chemistry and the biorefinery concept. Sustain. Chem. Process. 2013, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: Productively. Green Chem. 2005, 7, 761. [Google Scholar] [CrossRef]
- Turner, M.K. Biocatalysis in organic chemistry (Part II): Present and future. Trends Biotechnol. 1995, 13, 253–258. [Google Scholar] [CrossRef]
- Octave, S.; Thomas, D. Biorefinery: Toward an industrial metabolism. Biochimie 2009, 91, 659–664. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Ortiz, A.L.; Segura, F.N.; Jabalera, R.S.; Paula, M.M.D.; Del Campo, E.A.; Gutiérrez, J.S.; Bretado, M.E.; Collins-Martínez, V.; Bretado, M.A.E. Low temperature sugar cane bagasse pyrolysis for the production of high purity hydrogen through steam reforming and CO2 capture. Int. J. Hydrog. Energy 2013, 38, 12580–12588. [Google Scholar] [CrossRef]
- Hoi, L.W.S.; Martincigh, B.S. Sugar cane plant fibres: Separation and characterisation. Ind. Crop. Prod. 2013, 47, 1–12. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Rocha, I.M.; Galvão, T.L.P.; Sapei, E.; Da Silva, M.D.; Da Silva, M.A.V.R. Levoglucosan: A Calorimetric, Thermodynamic, Spectroscopic, and Computational Investigation. J. Chem. Eng. Data 2013, 58, 1813–1821. [Google Scholar] [CrossRef]
- Junior, I.I.; Flores, M.C.; Sutili, F.K.; Leite, S.G.F.; Leandro, L.S.; Leal, I.C.R.; De Souza, R.O. Lipase-catalyzed monostearin synthesis under continuous flow conditions. Org. Process Res. Dev. 2012, 16, 1098–1101. [Google Scholar] [CrossRef]
- Galletti, P.; Moretti, F.; Samori’, C.; Tagliavini, E. Enzymatic acylation of levoglucosan in acetonitrile and ionic liquids. Green Chem. 2007, 9, 987–991. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Da Pieve, S.; Marzona, S.; Nicoli, M.C. Effect of monoglyceride-oil–water gels on white bread properties. Food Res. Int. 2012, 49, 778–782. [Google Scholar] [CrossRef]
- Do Nascimento, M.A.; Gotardo, L.E.; Leão, R.A.C.; De Castro, A.M.; De Souza, R.O.; Itabaiana, I. Enhanced Productivity in Glycerol Carbonate Synthesis under Continuous Flow Conditions: Combination of Immobilized Lipases from Porcine Pancreas and Candida antarctica (CALB) on Epoxy Resins. ACS Omega 2019, 4, 860–869. [Google Scholar] [CrossRef] [Green Version]
- De Souza, S.P.; De Almeida, R.A.D.; Garcia, G.G.; Leão, R.A.C.; Bassut, J.; De Souza, R.O.M.A.; Itabaiana, I. Immobilization of lipase B from Candida antarctica on epoxy-functionalized silica: Characterization and improving biocatalytic parameters. J. Chem. Technol. Biotechnol. 2017, 93, 105–111. [Google Scholar] [CrossRef]
- Aguillón, A.R.; Avelar, M.N.; Gotardo, L.E.; De Souza, S.P.; Leão, Raquel, A.C.; Itabaiana, I.; Miranda Leandro, S.M.; De Souza, R.O.M.A. Immobilized lipase screening towards continuous-flow kinetic resolution of (±)-1,2-propanediol. Mol. Cat. 2019, 467, 128–134. [Google Scholar] [CrossRef]
- Fathi, Z.; Doustkhah, E.; Rostamnia, S.; Darvishi, F.; Ghodsi, A.; Ide, Y. Interaction of Yarrowia lipolytica lipase with dithiocarbamate modified magnetic carbon Fe 3 O 4 @C-NHCS 2 H core-shell nanoparticles. Int. J. Boil. Macromol. 2018, 117, 218–224. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Brask, J.; Mohammadi, M. Preparation of highly reusable biocatalysts by immobilization of lipases on epoxy-functionalized silica for production of biodiesel from canola oil. Biochem. Eng. J. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Zdráhal, Z.; Oliveira, J.; Vermeylen, R.; Claeys, M.; Maenhaut, W. Improved Method for Quantifying Levoglucosan and Related Monosaccharide Anhydrides in Atmospheric Aerosols and Application to Samples from Urban and Tropical Locations. Environ. Sci. Technol. 2002, 36, 747–753. [Google Scholar] [CrossRef]
- Schummer, C.; Delhomme, O.; Appenzeller, B.; Wennig, R.; Millet, M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 2009, 77, 1473–1482. [Google Scholar] [CrossRef]
- Trost, B.M. The Atom Economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Mateo, C.; Torres, R.T.R.; Fernandez-Lorente, G.; Ortiz, C.; Fuentes, M.; Hidalgo, A.; López-Gallego, F.; Abian, O.; Palomo, J.M.; Betancor, L.; et al. Epoxy-Amino Groups: A New Tool for Improved Immobilization of Proteins by the Epoxy Method. Biomacromolecules 2003, 4, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Boissièere-Junot, N.; Tellier, C.; Rabiller, C. On the Regioselective Acylation of 1,6-Anhydro-β- d—and l -Hexopyranoses Catalysed by Lipases: Structural Basis and Synthetic Applications. J. Carbohydr. Chem. 1998, 17, 99–115. [Google Scholar] [CrossRef]
- Rotticci, D. Understanding and Engineering the Enantioselectivity of Candida Antarctica Lipase B towards Sec–Alcohols. Ph.D. Thesis, Royal Institute of Technology, Department of Chemistry, Organic Chemistry, Stockholm, Sweden, 2000. [Google Scholar]
- Uriarte, I.; Écija, P.; Lozada-Garcia, R.; Çarçabal, P.; Cocinero, E.J. Investigating the Conformation of the Bridged Monosaccharide Levoglucosan. ChemPhysChem 2018, 19, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.K.; Hall, L.D.; Sanders, J.K.M. The Conformation of Vinblastine in Solution as Determined by N.O.E. Difference Spectroscopy. J. Chem. Soc. Perkin Trans. 1983, 1, 657–665. [Google Scholar] [CrossRef]







 | |||||
| Entry | Biocatalyst * | Acyl Donor ** | Conversion (%) (3-I + 3-II + 3-III) | ||
| 50 °C | 55 °C | 65 °C | |||
| 1 | N435 | a | 46 | 92 | 68 |
| 2 | b | 97 | 90 | 62 | |
| 3 | c | 58 | 84 | 31 | |
| 4 | d | 48 | 86 | 49 | |
| 5 | CaLB_epoxy | a | 49 | 29 | 68 |
| 6 | b | 76 | 70 | 64 | |
| 7 | c | 0 | 61 | 16 | |
| 8 | d | 12 | 93 | 77 | |
| 9 | PSIM | a | 51 | 52 | 12 |
| 10 | b | 49 | 74 | 51 | |
| 11 | c | 47 | 70 | 0 | |
| 12 | d | 8 | 72 | 67 | |
| 1 | 2 | |||
|---|---|---|---|---|
| Carbon/Hydrogen | 13C | 1H | 13C | 1H |
| 1 | 101.25 | 5.44 s | 102.27 | 5.51 s |
| 2 | 73.54 | 4.76 m | 70.17 | 3.57 m |
| 3 | 67.91 | 3.54 d (1.0 Hz) | 71.53 | 3.78 m |
| 4 | 68.86 | 3.61 s | 72.50 | 4.74 m |
| 5 | 76.01 | 4.58 d (5.4 Hz) | 74.54 | 4.58 d (4.9 Hz) |
| 6 | 64.88 | 4.06 d (H6 endo, 7.0 Hz) | 65.91 | 4.24 d (H6 endo, 7.7 Hz) |
| 3.81 dd (H6 exo, 7.4 and 5.9 Hz) | 3.81 dd (H6 exo, 7.6 and 5.7 Hz) | |||
| 2′ | 34.38 | 2.34 t (7.5 Hz) | 34.4 | 2.39 t (7.6 Hz) |
| 3′ | 24.77 | 1.64 m | 25.04 | 1.64 m |
| 4′-7 and 12′-17′ | 29.18–29.90 | 1.28 m | 29.19–29.92 | 1.29 m |
| 8′ and 11′ | 27.16 and 27.23 | 2.0 m | 27.31 and 27.38 | 2.02 m |
| 9′ and 10′ | 129.82–130.21 | 5.34 m | 130.2–129.86 | 5.34 m |
| 18’ | 14.25 | 0.87 t (6.9 Hz) | 14.24 | 0.88 t (6.6 Hz) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avelar do Nascimento, M.; Ester Gotardo, L.; Miguez Bastos, E.; C. L. Almeida, F.; A. C. Leão, R.; O. M. A. de Souza, R.; Wojcieszak, R.; Itabaiana, I., Jr. Regioselective Acylation of Levoglucosan Catalyzed by Candida Antarctica (CaLB) Lipase Immobilized on Epoxy Resin. Sustainability 2019, 11, 6044. https://doi.org/10.3390/su11216044
Avelar do Nascimento M, Ester Gotardo L, Miguez Bastos E, C. L. Almeida F, A. C. Leão R, O. M. A. de Souza R, Wojcieszak R, Itabaiana I Jr. Regioselective Acylation of Levoglucosan Catalyzed by Candida Antarctica (CaLB) Lipase Immobilized on Epoxy Resin. Sustainability. 2019; 11(21):6044. https://doi.org/10.3390/su11216044
Chicago/Turabian StyleAvelar do Nascimento, Marcelo, Larissa Ester Gotardo, Eduardo Miguez Bastos, Fabio C. L. Almeida, Raquel A. C. Leão, Rodrigo O. M. A. de Souza, Robert Wojcieszak, and Ivaldo Itabaiana, Jr. 2019. "Regioselective Acylation of Levoglucosan Catalyzed by Candida Antarctica (CaLB) Lipase Immobilized on Epoxy Resin" Sustainability 11, no. 21: 6044. https://doi.org/10.3390/su11216044
APA StyleAvelar do Nascimento, M., Ester Gotardo, L., Miguez Bastos, E., C. L. Almeida, F., A. C. Leão, R., O. M. A. de Souza, R., Wojcieszak, R., & Itabaiana, I., Jr. (2019). Regioselective Acylation of Levoglucosan Catalyzed by Candida Antarctica (CaLB) Lipase Immobilized on Epoxy Resin. Sustainability, 11(21), 6044. https://doi.org/10.3390/su11216044