Slow-Release Urea Prills Developed Using Organic and Inorganic Blends in Fluidized Bed Coater and Their Effect on Spinach Productivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Spray Coating Solutions
2.3. Apparatus
2.4. Scanning Electron Microscopy (SEM)
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. X-ray Diffraction
2.7. Crushing Strength
2.8. UV- Visible Spectrophotometry
2.9. Test Protocol
2.10. Pot Experiment
2.11. Release Kinetics
Urea Release Kinetics
2.12. Statistical Analysis
3. Results and Discussion
3.1. Scanning Electron Microscope
Effect of Coating on Surface Morphology
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3. X-ray Diffraction (XRD)
3.4. Crushing Strength
Effect of Coating on the Crushing Strength
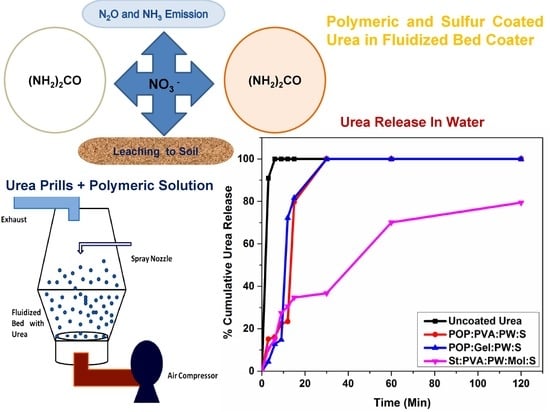
3.5. Effect of Coating on the Rate of Urea–Nitrogen Release
3.6. Spinach Biomass Yield and N Uptake
3.7. Release Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Symbols | |
| SEM | scanning electron microscope |
| UV—VIS | Ultraviolet Visible (UV) spectroscopy |
| FTIR | Fourier transform infra-red |
| XRD | X-ray diffraction |
| POP | plaster of Paris |
| PVA | poly vinyl alcohol |
| N | Nitrogen |
| C-0 | Uncoated urea |
| C-1 | urea coated with PVA 5%, plaster of Paris 10%, sulfur 5%, paraffin wax 2% |
| C-2 | urea coated with PVA 5%, starch 10%, sulfur 5%, paraffin wax 2% |
| C-3 | urea coated with gelatin 5%, plaster of Paris 10%, sulfur 5%, paraffin wax 2% |
| C-4 | urea coated with PVA 5%, starch 10%, sulfur 5%, paraffin wax 2.5%, molasses 2.5% |
References
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Brady, N.C.; Weil, R.R. Soil Colloids: Seat of Soil Chemical and Physical Acidity; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2008; pp. 311–358. [Google Scholar]
- Matson, P.A.; Naylor, R.; Ortiz-Monasterio, I. Integration of environmental, agronomic, and economic aspects of fertilizer management. Science 1998, 280, 112–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, D.B.; Torbert, H.A.; Prior, S.A.; Huluka, G. Long-term tillage and poultry litter impacts soil carbon and nitrogen mineralization and fertility. Soil Sci. Soc. Am. J. 2010, 74, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.A.; Ali, A.; Tahir, M.; Naeem, M.; Chadhar, A.R.; Ahmad, S. Effect of nitrogen levels and plant spacing on growth and yield of cotton. Pak. J. Life Soc. Sci. 2010, 8, 121–124. [Google Scholar]
- Fan, X.H.; Li, Y.C. Nitrogen release from slow-release fertilizers as affected by soil type and temperature. Soil Sci. Soc. Am. J. 2010, 74, 1635–1641. [Google Scholar] [CrossRef]
- Follett, R.F. Fate and Transport of Nutrients: Nitrogen; US Department of Agriculture, Soil Conservation Service: Fort Collins, CO, USA, 1995. [Google Scholar]
- Jain, V.; Abrol, Y. Plant Nitrogen Use Efficiency. In The Indian Nitrogen Assessment; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–173. [Google Scholar]
- Rahmanian, N.; Naderi, S.; Supuk, E.; Abbas, R.; Hassanpour, A. Urea finishing process: Prilling versus granulation. Procedia Eng. 2015, 102, 174–181. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R. Bioactive Foods in Promoting Health: Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Mohajan, H. Planetary Boundaries Must not be Crossed for the Survival of Humanity. J. Environ. Treat. Tech. 2015, 3, 184–200. [Google Scholar]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating materials for slow release of nitrogen from urea fertilizer: A review. J. Plant Nutr. 2020, 43, 1510–1533. [Google Scholar] [CrossRef]
- Trenkel, M.E. Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association: Paris, France, 1997; Volume 11. [Google Scholar]
- Al-Zahrani, S.M. Controlled-release of fertilizers: Modelling and simulation. Int. J. Eng. Sci. 1999, 37, 1299–1307. [Google Scholar] [CrossRef]
- Kent, J.A. Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology; Springer US: New York, NY, USA, 2007. [Google Scholar]
- Wang, X.; Lü, S.; Gao, C.; Xu, X.; Zhang, X.; Bai, X.; Liu, M.; Wu, L. Highly efficient adsorption of ammonium onto palygorskite nanocomposite and evaluation of its recovery as a multifunctional slow-release fertilizer. Chem. Eng. J. 2014, 252, 404–414. [Google Scholar] [CrossRef]
- Vudjung, C.; Saengsuwan, S. Biodegradable IPN hydrogels based on pre-vulcanized natural rubber and cassava starch as coating membrane for environment-friendly slow-release urea fertilizer. J. Polym. Environ. 2018, 26, 3967–3980. [Google Scholar] [CrossRef]
- Ibrahim, K.R.M.; Babadi, F.E.; Yunus, R. Comparative performance of different urea coating materials for slow release. Particuology 2014, 17, 165–172. [Google Scholar] [CrossRef]
- Salman, O.A. Polyethylene-coated urea. 1. Improved storage and handling properties. Ind. Eng. Chem. Res. 1989, 28, 630–632. [Google Scholar] [CrossRef]
- Babadi, F.E.; Yunus, R.; Rashid, S.A.; Salleh, M.A.M.; Ali, S. New coating formulation for the slow release of urea using a mixture of gypsum and dolomitic limestone. Particuology 2015, 23, 62–67. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Ozturk, L.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef]
- Barker, A.V.; Maynard, D.N.; Mills, H.A. Variations in nitrate accumulation among spinach cultivars. J. Am. Soc. Hortic. Sci. 1974, 99, 132–134. [Google Scholar]
- Kjeldahl, J.G.C.T. Neue methode zur bestimmung des stickstoffs in organischen körpern. Eitschrift Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Datta, U.; Das, R.; Chakraborty, K. Comparative Assessment of Leaf Chlorophyll Content of Seven Selected Vegetable Crop by Two Alternative Methods at Murshidabad, West Bengal. Int. J. Pharm. Biol. Sci. 2018, 8, 570–578. [Google Scholar]
- Al-Zahrani, S.M. Utilization of polyethylene and paraffin waxes as controlled delivery systems for different fertilizers. Ind. Eng. Chem. Res. 2000, 39, 367–371. [Google Scholar] [CrossRef]
- Lubkowski, K. Coating fertilizer granules with biodegradable materials for controlled fertilizer release. Environ. Eng. Manag. J. 2014, 13, 2573–2581. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Testing of starch-based carbohydrate polymer coatings for enhanced urea performance. J. Coat. Technol. Res. 2014, 11, 747–756. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef] [PubMed]
- De Colli, M.; Massimi, M.; Barbetta, A.; Di Rosario, B.L.; Nardecchia, S.; Devirgiliis, L.C.; Dentini, M. A biomimetic porous hydrogel of gelatin and glycosaminoglycans cross-linked with transglutaminase and its application in the culture of hepatocytes. Biomed. Mater. 2012, 7, 055005. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S.; Lue, Y.Y.; Mohd Ishak, Z.A. Toughening of Poly (Lactic Acid) Nanocomposites. Available online: https://vdokumen.net/reader/full/buletin-pusat-pengajian-kejuruteraan-bahan-dan-sumber-pameran-poster-latihan (accessed on 2 June 2020).
- Noriman, N.Z.; Ismail, H.; Rashid, A.A. Properties of styrene butadiene rubber/recycled acrylonitrile-butadiene rubber (SBR/NBRr) blends: Effect of the addition of trans-polyoctylene rubber. J. Appl. Polym. Sci. 2012, 126, E56–E63. [Google Scholar] [CrossRef]
- Tan, W.L.; Bakar, M.A. Bakar, and compounds, Synthesis, characterization and impedance spectroscopy study of magnetite/epoxidized natural rubber nanocomposites. J. Alloys Compd. 2013, 561, 40–47. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Amaratunga, G.A. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Akmal, D. Use of bioblend polystyrene/starch for coating urea granules as slow release fertilizer. J. Chem. Pharm. Res. 2015, 7, 478–484. [Google Scholar]
- Roshanravan, B.; Soltani, S.M.; Rashid, S.A.; Mahdavi, F.; Yusop, M.K. Enhancement of nitrogen release properties of urea–kaolinite fertilizer with chitosan binder. Chem. Speciat. Bioavailab. 2015, 27, 44–51. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2014. [Google Scholar]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Pogrebnoi, A.; Shao, G.; Hilonga, A. Encapsulated urea-kaolinite nanocomposite for controlled release fertilizer formulations. J. Chem. 2015, 2015, 237397. [Google Scholar] [CrossRef]
- Aleksandrova, E.A.; Aleksandrov, B.L.; Khadisova, Z.T.; Krasavtsev, B.E. Structural and Mechanical Properties of Paraffin Wax Composites. Chem. Technol. Fuels Oils 2018, 54, 37–43. [Google Scholar] [CrossRef]
- Prodpran, T.; Benjakul, S.; Vittayanont, M.; Nalinanon, S. Physico-chemical properties of gelatin films incorporated with different hydrocolloids. Int. Proc. Chem. Biol. Environ. Eng. 2013, 53, 82–86. [Google Scholar]
- Trenkel, M.E. Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Efficiency in Agriculture, 2nd ed.; IFA: Paris, France, 2010; ISBN 978-2-9523139-7-1. [Google Scholar]
- Li, Z.; Zhang, Y.; Li, Y. Zeolite as slow release fertilizer on spinach yields and quality in a greenhouse test. J. Plant Nutr. 2013, 36, 1496–1505. [Google Scholar] [CrossRef]
- Rodríguez-Félix, D.E.; Pérez-Martínez, C.J.; Castillo-Ortega, M.M.; Pérez-Tello, M.; Romero-García, J.; Ledezma-Pérez, A.S.; Del Castillo-Castro, T.; Rodríguez-Félix, F. pH-and temperature-sensitive semi-interpenetrating network hydrogels composed of poly (acrylamide) and poly (γ-glutamic acid) as amoxicillin controlled-release system. Polym. Bull. 2012, 68, 197–207. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]








| Treatment Code | Sulphur | Starch | PVA * | POP ** | Molasses | Gelatin | Paraffin Wax |
|---|---|---|---|---|---|---|---|
| (g (100 g of Urea)−1) | |||||||
| C-1 | 5 | - | 5 | 10 | - | - | 2 |
| C-2 | 5 | 10 | 5 | - | - | - | 2 |
| C-3 | 5 | - | - | 10 | - | 5 | 2 |
| C-4 | 5 | 10 | 5 | - | 2.5 | - | 2.5 |
| Model Equation | Sample Name | Adjusted R Square | Value of “k” | Value of “n” | Reduced Chi-Squared |
|---|---|---|---|---|---|
| Qt = ktn | uncoated | 0.2072 | 0.947 | 0.015 | 0.0008 |
| C-1 | 0.6332 | 0.160 | 0.417 | 0.0619 | |
| C-2 | 0.6197 | 0.317 | 0.269 | 0.0383 | |
| C-3 | 0.5680 | 0.192 | 0.381 | 0.0787 | |
| C-4 | 0.6582 | 0.387 | 0.321 | 0.0541 |
| Model Name | Sample Name | Adjusted R Square | Value of “a” | Value of “b” | Reduced Chi-Squared |
|---|---|---|---|---|---|
| Qt = at/1+bt modified hyperbola | uncoated | 0.9975 | 3.709 | 3.652 | 0.0002 |
| C-1 | 0.8183 | 0.060 | 0.045 | 0.0334 | |
| C-2 | 0.8824 | 0.125 | 0.107 | 0.0169 | |
| C-3 | 0.7985 | 0.075 | 0.058 | 0.0404 | |
| C-4 | 0.8912 | 0.112 | 0.099 | 0.0289 |
| Model Name | Sample Name | Adjusted R Square | Value of “b” | Reduced Chi-Squared |
|---|---|---|---|---|
| Qt = (1−e−bt) Schwartz and Sinclair formula | uncoated | 0.9999 | 0.8043 | 0.000008 |
| C-1 | 0.8657 | 0.054 | 0.0247 | |
| C-2 | 0.9239 | 0.093 | 0.0109 | |
| C-3 | 0.8548 | 0.066 | 0.0291 | |
| C-4 | 0.9192 | 0.084 | 0.0201 |
| Model Name | Sample Name | Adjusted R Square | Value of “a” | Value of “b” | Reduced Chi-Squared |
|---|---|---|---|---|---|
| Qt = a(1−e−bt) modified Schwartz and Sinclair formula | uncoated | 0.9999 | 1.001 | 0.7999 | 0.000008 |
| C-1 | 0.8532 | 1.070 | 0.0484 | 0.02699 | |
| C-2 | 0.9156 | 1.032 | 0.0881 | 0.01213 | |
| C-3 | 0.8398 | 1.062 | 0.0599 | 0.03211 | |
| C-4 | 0.9011 | 1.041 | 0.0679 | 0.02013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beig, B.; Niazi, M.B.K.; Jahan, Z.; Pervaiz, E.; Abbas Shah, G.; Ul Haq, M.; Zafar, M.I.; Zia, M. Slow-Release Urea Prills Developed Using Organic and Inorganic Blends in Fluidized Bed Coater and Their Effect on Spinach Productivity. Sustainability 2020, 12, 5944. https://doi.org/10.3390/su12155944
Beig B, Niazi MBK, Jahan Z, Pervaiz E, Abbas Shah G, Ul Haq M, Zafar MI, Zia M. Slow-Release Urea Prills Developed Using Organic and Inorganic Blends in Fluidized Bed Coater and Their Effect on Spinach Productivity. Sustainability. 2020; 12(15):5944. https://doi.org/10.3390/su12155944
Chicago/Turabian StyleBeig, Bilal, Muhammad Bilal Khan Niazi, Zaib Jahan, Erum Pervaiz, Ghulam Abbas Shah, Midrar Ul Haq, Mazhar Iqbal Zafar, and Munir Zia. 2020. "Slow-Release Urea Prills Developed Using Organic and Inorganic Blends in Fluidized Bed Coater and Their Effect on Spinach Productivity" Sustainability 12, no. 15: 5944. https://doi.org/10.3390/su12155944
APA StyleBeig, B., Niazi, M. B. K., Jahan, Z., Pervaiz, E., Abbas Shah, G., Ul Haq, M., Zafar, M. I., & Zia, M. (2020). Slow-Release Urea Prills Developed Using Organic and Inorganic Blends in Fluidized Bed Coater and Their Effect on Spinach Productivity. Sustainability, 12(15), 5944. https://doi.org/10.3390/su12155944