Temporal Variation and Spatial Distribution in the Water Environment Helps Explain Seasonal Dynamics of Zooplankton in River-Type Reservoir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Sampling
2.3. Data Processing
3. Results
3.1. Spatial and Temporal Heterogeneity of Reservoir’s Water Environment
3.1.1. Physicochemical Indicators of Water Bodies
3.1.2. Biological Indicators of Water Bodies
3.1.3. Pollution Indicators of Water Bodies
3.2. Spatial and Temporal Heterogeneity of Reservoir’s Zooplankton
3.3. Relationships between Water Environmental Factors and Zooplankton
3.3.1. Principal Component Analysis (PCA)
3.3.2. Redundancy and Correlation Analysis
4. Discussion
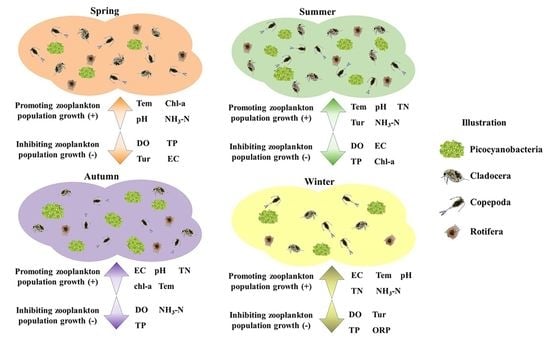
4.1. Factors Influencing Zooplankton Distribution
4.2. Variation of Zooplankton Distribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a Cultivated Planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary Boundaries: Guiding Human Development on a Changing Planet. Science 2015, 347, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.R.; Busquets, R.; Campos, L.C. Assessment of Microplastics in Freshwater Systems: A Review. Sci. Total Environ. 2020, 707, 12. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Alonso, C.; Alvarez, X.; Lorenzo, O.; Cancela, A.; Valero, E.; Sanchez, A. Water Toxicity in Reservoirs after Freshwater Algae Harvest. J. Clean. Prod. 2021, 283, 104560. [Google Scholar] [CrossRef]
- Soares, L.M.V.; Calijuri, M.D. Deterministic Modelling of Freshwater Lakes and Reservoirs: Current Trends and Recent Progress. Environ. Model. Softw. 2021, 144, 16. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhang, Z.; Xue, S.; Wang, R.J.; Xiao, M. Stability Analysis of a Typical Landslide Mass in the Three Gorges Reservoir under Varying Reservoir Water Levels. Environ. Earth Sci. 2020, 79, 14. [Google Scholar] [CrossRef]
- Hossain, M.; Huda, A.S.N.; Mekhilef, S.; Seyedmahmoudian, M.; Horan, B.; Stojcevski, A.; Ahmed, M. A State-of-the-Art Review of Hydropower in Malaysia as Renewable Energy: Current Status and Future Prospects. Energy Strategy Rev. 2018, 22, 426–437. [Google Scholar] [CrossRef]
- Kosek, K.; Ruman, M. Arctic Freshwater Environment Altered by the Accumulation of Commonly Determined and Potentially New Pops. Water 2021, 13, 1739. [Google Scholar] [CrossRef]
- Ji, B.; Liang, J.C.; Chen, R. Bacterial Eutrophic Index for Potential Water Quality Evaluation of a Freshwater Ecosystem. Environ. Sci. Pollut. Res. 2020, 27, 32449–32455. [Google Scholar] [CrossRef]
- Park, Y.; Lee, H.K.; Shin, J.K.; Chon, K.; Kim, S.; Cho, K.H.; Kim, J.H.; Baek, S.S. A Machine Learning Approach for Early Warning of Cyanobacterial Bloom Outbreaks in a Freshwater Reservoir. J. Environ. Manag. 2021, 288, 9. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Ait-Aissa, S.; Burgess, R.M.; Busch, W.; Creusot, N.; Di Paolo, C.; Escher, B.I.; Hewitt, L.M.; Hilscherova, K.; Hollender, J.; et al. Effect-Directed Analysis Supporting Monitoring of Aquatic Environments—An in-Depth Overview. Sci. Total Environ. 2016, 544, 1073–1118. [Google Scholar] [CrossRef] [PubMed]
- Altenburger, R.; Brack, W.; Burgess, R.M.; Busch, W.; Escher, B.I.; Focks, A.; Hewitt, L.M.; Jacobsen, B.N.; de Alda, M.L.; Ait-Aissa, S.; et al. Future Water Quality Monitoring: Improving the Balance between Exposure and Toxicity Assessments of Real-World Pollutant Mixtures. Environ. Sci. Eur. 2019, 31, 17. [Google Scholar] [CrossRef] [Green Version]
- Sagan, V.; Peterson, K.T.; Maimaitijiang, M.; Sidike, P.; Sloan, J.; Greeling, B.A.; Maalouf, S.; Adams, C. Monitoring Inland Water Quality Using Remote Sensing: Potential and Limitations of Spectral Indices, Bio-Optical Simulations, Machine Learning, and Cloud Computing. Earth-Sci. Rev. 2020, 205, 31. [Google Scholar] [CrossRef]
- Shi, W.X.; Zhuang, W.E.; Hur, J.; Yang, L.Y. Monitoring Dissolved Organic Matter in Wastewater and Drinking Water Treatments Using Spectroscopic Analysis and Ultra-High Resolution Mass Spectrometry. Water Res. 2021, 188, 16. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.L.; Dodson, S.I. Predation, Body Size, and Composition of Plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional Constraints in Terrestrial and Freshwater Food Webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Frank, K.T.; Petrie, B.; Choi, J.S.; Leggett, W.C. Trophic Cascades in a Formerly Cod-Dominated Ecosystem. Science 2005, 308, 1621–1623. [Google Scholar] [CrossRef] [Green Version]
- Sousa, W.; Attayde, J.L.; Rocha, E.D.; Eskinazi-Sant’Anna, E.M. The Response of Zooplankton Assemblages to Variations in the Water Quality of Four Man-Made Lakes in Semi-Arid Northeastern Brazil. J. Plankton Res. 2008, 30, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Noges, P.; Davidson, T.A.; Haberman, J.; Noges, T.; Blank, K.; Lauridsen, T.L.; Sondergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes According to the European Water Framework Directive (Wfd). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Winder, M.; Schindler, D.E. Climate Change Uncouples Trophic Interactions in an Aquatic Ecosystem. Ecology 2004, 85, 2100–2106. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.A.; Sarnelle, O.; Wilson, A.E.; Lurling, M. The Interaction between Cyanobacteria and Zooplankton in a More Eutrophic World. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, B.; Clavero, M.; Sanchez, M.I.; Vila, M. Global Ecological Impacts of Invasive Species in Aquatic Ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Noges, T.; Noges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological Impacts of Global Warming and Water Abstraction on Lakes and Reservoirs Due to Changes in Water Level and Related Changes in Salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Miloslavich, P.; Bax, N.J.; Simmons, S.E.; Klein, E.; Appeltans, W.; Aburto-Oropeza, O.; Garcia, M.A.; Batten, S.D.; Benedetti-Cecchi, L.; Checkley, D.M.; et al. Essential Ocean Variables for Global Sustained Observations of Biodiversity and Ecosystem Changes. Glob. Chang. Biol. 2018, 24, 2416–2433. [Google Scholar] [CrossRef] [Green Version]
- Marcolin, C.R.; Lopes, R.M.; Jackson, G.A. Estimating Zooplankton Vertical Distribution from Combined Lopc and Zooscan Observations on the Brazilian Coast. Mar. Biol. 2015, 162, 2171–2186. [Google Scholar] [CrossRef]
- Wang, W.C.; Sun, S.; Zhang, F.; Sun, X.X.; Zhang, G.T. Zooplankton Community Structure, Abundance and Biovolume in Jiaozhou Bay and the Adjacent Coastal Yellow Sea During Summers of 2005-2012: Relationships with Increasing Water Temperature. J. Oceanol. Limnol. 2018, 36, 1655–1670. [Google Scholar] [CrossRef]
- Naito, A.; Abe, Y.; Matsuno, K.; Nishizawa, B.; Kanna, N.; Sugiyama, S.; Yamaguchi, A. Surface Zooplankton Size and Taxonomic Composition in Bow Doi N Fjord, North-Western Greenland: A Comparison of Zooscan, Opc and Microscopic Analyses. Polar Sci. 2019, 19, 120–129. [Google Scholar] [CrossRef]
- Wang, W.C.; Sun, S.; Sun, X.X.; Zhang, G.T.; Zhang, F. Spatial Patterns of Zooplankton Size Structure in Relation to Environmental Factors in Jiaozhou Bay, South Yellow Sea. Mar. Pollut. Bull. 2020, 150, 10. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.E.; Gossner, H.; Smith, M.J.; Blanco-Bercial, L.; Irigoien, X. Use of Optical Imaging Datasets to Assess Biogeochemical Contributions of the Mesozooplankton. J. Plankton Res. 2021, 43, 475–491. [Google Scholar] [CrossRef]
- Noyon, M.; Poulton, A.J.; Asdar, S.; Weitz, R.; Giering, S.L.C. Mesozooplankton Community Distribution on the Agulhas Bank in Autumn: Size Structure and Production. Deep-Sea Res. Part Ii-Top. Stud. Oceanogr. 2022, 195, 15. [Google Scholar] [CrossRef]
- Garcia-Herrera, N.; Cornils, A.; Laudien, J.; Niehoff, B.; Hofer, J.; Forsterra, G.; Gonzalez, H.E.; Richter, C. Seasonal and Diel Variations in the Vertical Distribution, Composition, Abundance and Biomass of Zooplankton in a Deep Chilean Patagonian Fjord. Peerj 2022, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Schultes, S.; Lopes, R.M. Laser Optical Plankton Counter and Zooscan Intercomparison in Tropical and Subtropical Marine Ecosystems. Limnol. Oceanogr.-Methods 2009, 7, 771–784. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, H. Calculation and Study of Total Nitrogen Influx Fluxes in Tributaries of Shanxi Reservoir. Zhejiang Hydrotech. 2017, 45, 22–24. [Google Scholar] [CrossRef]
- Li, A.L.; Haitao, C.; Yuanyuan, L.; Qiu, L.; Wenchuan, W. Simulation of Nitrogen Pollution in the Shanxi Reservoir Watershed Based on Swat Model. Nat. Environ. Pollut. Technol. 2020, 19, 1265–1272. [Google Scholar] [CrossRef]
- Chen, H.T.; Chen, J.; Liu, Y.Y.; He, J. Study of Nitrogen Pollution Simulation and Management Measures on Swat Model in Typhoon Period of Shanxi Reservoir Watershed, Zhejiang Province, China. Pol. J. Environ. Stud. 2021, 30, 2499–2507. [Google Scholar] [CrossRef]
- Yang, M.Z.; Xia, J.H.; Cai, W.W.; Zhou, Z.Y.; Yang, L.B.; Zhu, X.X.; Li, C.D. Seasonal and Spatial Distributions of Morpho-Functional Phytoplankton Groups and the Role of Environmental Factors in a Subtropical River-Type Reservoir. Water Sci. Technol. 2020, 82, 2316–2330. [Google Scholar] [CrossRef] [PubMed]
- Pianka, E.R. Niche Overlap and Diffuse Competition. Proc. Natl. Acad. Sci. USA 1974, 71, 2141–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, W.; Huang, X.N.; Chen, Y.Y.; Fu, R.Y.; Du, X.; Chen, X.Y.; Zhan, A.B. Zooplankton Biodiversity Monitoring in Polluted Freshwater Ecosystems: A Technical Review. Environ. Sci. Ecotechnol. 2020, 1, 11. [Google Scholar] [CrossRef]
- Warren, J.D.; Leach, T.H.; Williamson, C.E. Measuring the Distribution, Abundance, and Biovolume of Zooplankton in an Oligotrophic Freshwater Lake with a 710 Khz Scientific Echosounder. Limnol. Oceanogr.-Methods 2016, 14, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Sastri, A.R.; Gauthier, J.; Juneau, P.; Beisner, B.E. Biomass and Productivity Responses of Zooplankton Communities to Experimental Thermocline Deepening. Limnol. Oceanogr. 2014, 59, 1–16. [Google Scholar] [CrossRef]
- Gauthier, J.; Prairie, Y.T.; Beisner, B.E. Thermocline Deepening and Mixing Alter Zooplankton Phenology, Biomass and Body Size in a Whole-Lake Experiment. Freshw. Biol. 2014, 59, 998–1011. [Google Scholar] [CrossRef]
- Yaseen, T.; Bhat, S.U.; Bhat, F.A. Study of Vertical Distribution Dynamics of Zooplankton in a Thermally Stratified Warm Monomictic Lake of Kashmir Himalaya. Ecohydrology 2022, 15, 18. [Google Scholar] [CrossRef]
- GB11892-89; Water Quality-Determination of Permanganate index. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 1989. (In Chinese)
- GB11893-89; Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 1989. (In Chinese)
- HJ535-2009; Water Quality-Determination of Ammonia Nitrogen-Nessler’s Reagent Spectrophotometry. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2009. (In Chinese)
- SCT9402-2010; Specifications for Freshwater Plankton Surveys. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2010. (In Chinese)
- Vogelmann, C.; Teichert, M.; Schubert, M.; Martens, A.; Schultes, S.; Stibor, H. The Usage of a Zooplankton Digitization Software to Study Plankton Dynamics in Freshwater Fisheries. Fish. Res. 2022, 251, 9. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Singh, T.P.; Batish, A. On Mechanical Characterization of 3-D Printed Pla-Pvc-Wood Dust-Fe3o4 Composite. J. Thermoplast. Compos. Mater. 2022, 35, 36–53. [Google Scholar] [CrossRef]
- Sharma, A.S.; Gupta, S.; Singh, N.R. Zooplankton Community of Keibul Lamjao National Park (Klnp) Manipur, India in Relation to the Physico-Chemical Variables of the Water. Chin. J. Oceanol. Limnol. 2017, 35, 469–480. [Google Scholar] [CrossRef]
- Xiang, R.; Wang, L.J.; Li, H.; Tian, Z.B.; Zheng, B.H. Water Quality Variation in Tributaries of the Three Gorges Reservoir from 2000 to 2015. Water Res. 2021, 195, 12. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Zheng, S.; Guo, S.J.; Zhu, M.L.; Liang, J.H.; Du, J.; Sun, X.X. Relationship between Zooplankton Community Characteristics and Environmental Conditions in the Surface Waters of the Western Pacific Ocean During the Winter of 2014. J. Ocean Univ. China 2021, 20, 706–720. [Google Scholar] [CrossRef]
- Lima, A.R.A.; Costa, M.F.; Barletta, M. Distribution Patterns of Microplastics within the Plankton of a Tropical Estuary. Environ. Res. 2014, 132, 146–155. [Google Scholar] [CrossRef]
- Wu, S.; Hua, P.; Gui, D.; Zhang, J.; Ying, G.; Krebs, P. Occurrences, Transport Drivers, and Risk Assessments of Antibiotics in Typical Oasis Surface and Groundwater. Water Res. 2022, 225, 119138. [Google Scholar] [CrossRef]
- Committee, E. Atlas of the Main Freshwater Zooplankton of Zhejiang Province (Drinking Water Sources); China Environment Publishing House: Beijing, China, 2013; Volume 445. (In Chinese) [Google Scholar]
- Olsen, R.L.; Chappell, R.W.; Loftis, J.C. Water Quality Sample Collection, Data Treatment and Results Presentation for Principal Components Analysis—Literature Review and Illinois River Watershed Case Study. Water Res. 2012, 46, 3110–3122. [Google Scholar] [CrossRef]
- ZerfaSs, C.; Lehmann, R.; Ueberschaar, N.; Sanchez-Arcos, C.; Totsche, K.U.; Pohnert, G. Groundwater Metabolome Responds to Recharge in Fractured Sedimentary Strata. Water Res. 2022, 223, 118998. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, Y.; Zhang, W.L.; Lin, L.; Qian, B.; Wang, L.F.; Niu, L.H.; Zhang, H.J. Cascade Dam Impoundments Restrain the Trophic Transfer Efficiencies in Benthic Microbial Food Web. Water Res. 2020, 170, 115351. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, J.; Xiao, Z.J. Investigation on River Thermal Regime under Dam Influence by Integrating Remote Sensing and Water Temperature Model. Water 2021, 13, 133. [Google Scholar] [CrossRef]
- Yao, L.G.; Zhao, X.M.; Zhou, G.J.; Liang, R.C.; Gou, T.; Xia, B.C.; Li, S.Y.; Liu, C. Seasonal Succession of Phytoplankton Functional Groups and Driving Factors of Cyanobacterial Blooms in a Subtropical Reservoir in South China. Water 2020, 12, 1167. [Google Scholar] [CrossRef] [Green Version]
- Akis, S.; Ozcimen, D. Optimization of Ph Induced Flocculation of Marine and Freshwater Microalgae Via Central Composite Design. Biotechnol. Prog. 2019, 35, 6. [Google Scholar] [CrossRef]
- Gollnisch, R.; Alling, T.; Stockenreiter, M.; Ahren, D.; Grabowska, M.; Rengefors, K. Calcium and Ph Interaction Limits Bloom Formation and Expansion of a Nuisance Microalga. Limnol. Oceanogr. 2021, 66, 3523–3534. [Google Scholar] [CrossRef]
- Price, G.A.V.; Stauber, J.L.; Holland, A.; Koppel, D.J.; Van Genderen, E.J.; Ryan, A.C.; Jolley, D.F. The Influence of Ph on Zinc Lability and Toxicity to a Tropical Freshwater Microalga. Environ. Toxicol. Chem. 2021, 40, 2836–2845. [Google Scholar] [CrossRef]
- Assuncao, A.W.D.; Souza, B.P.; da Cunha-Santino, M.B.; Bianchini, I. Formation and Mineralization Kinetics of Dissolved Humic Substances from Aquatic Macrophytes Decomposition. J. Soils Sediments 2018, 18, 1252–1264. [Google Scholar] [CrossRef]
- Sosa-Aranda, I.; Zambrano, L. Relationship between Turbidity and the Benthic Community in the Preserved Montebello Lakes in Chiapas, Mexico. Mar. Freshw. Res. 2020, 71, 824–831. [Google Scholar] [CrossRef]
- Gozdziejewska, A.M.; Kruk, M. Zooplankton Network Conditioned by Turbidity Gradient in Small Anthropogenic Reservoirs. Sci. Rep. 2022, 12, 12. [Google Scholar] [CrossRef]
- Vad, C.F.; Horvath, Z.; Kiss, K.T.; Toth, B.; Pentek, A.L.; Acs, E. Vertical Distribution of Zooplankton in a Shallow Peatland Pond: The Limiting Role of Dissolved Oxygen. Ann. Limnol.-Int. J. Limnol. 2013, 49, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Dan, S.F.; Liu, Y.; He, J.; Zhu, D.D.; Jiao, L.X. Importance of Ammonia Nitrogen Potentially Released from Sediments to the Development of Eutrophication in a Plateau Lake. Environ. Pollut. 2022, 305, 11. [Google Scholar] [CrossRef] [PubMed]
- Pulsifer, J.; Laws, E. Temperature Dependence of Freshwater Phytoplankton Growth Rates and Zooplankton Grazing Rates. Water 2021, 13, 1591. [Google Scholar] [CrossRef]
- Amorim, C.A.; Moura, A.D. Ecological Impacts of Freshwater Algal Blooms on Water Quality, Plankton Biodiversity, Structure, and Ecosystem Functioning. Sci. Total Environ. 2021, 758, 13. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.W.; Galbraith, L.M. Relating Planktonic Microbial Food Web Structure in Lentic Freshwater Ecosystems to Water Quality and Land Use. J. Plankton Res. 2007, 29, 127–139. [Google Scholar] [CrossRef]
- Li, C.C.; Feng, W.Y.; Chen, H.Y.; Li, X.F.; Song, F.H.; Guo, W.J.; Giesy, J.P.; Sun, F.H. Temporal Variation in Zooplankton and Phytoplankton Community Species Composition and the Affecting Factors in Lake Taihu-a Large Freshwater Lake in China. Environ. Pollut. 2019, 245, 1050–1057. [Google Scholar] [CrossRef]
- Huang, J.Y.; Wang, X.; Wang, X.Y.; Chen, Y.J.; Yang, Z.W.; Xie, S.G.; Li, T.T.; Song, S. Distribution Characteristics of Ammonia-Oxidizing Microorganisms and Their Responses to External Nitrogen and Carbon in Sediments of a Freshwater Reservoir, China. Aquat. Ecol. 2022, 56, 841–857. [Google Scholar] [CrossRef]
- Rhode, S.C.; Pawlowski, M.; Tollrian, R. The Impact of Ultraviolet Radiation on the Vertical Distribution of Zooplankton of the Genus Daphnia. Nature 2001, 412, 69–72. [Google Scholar] [CrossRef]
- Diniz, L.P.; Franca, E.J.; Bonecker, C.C.; Marcolin, C.R.; De Melo, M. Non-Predatory Mortality of Planktonic Microcrustaceans (Cladocera and Copepoda) in Neotropical Semiarid Reservoirs. An. Acad. Bras. Cienc. 2021, 93, 16. [Google Scholar] [CrossRef]









| Region | Shanxi Reservoir (SR) | Huangtankeng Stream (HS) | Xuezuokou Stream (XS) | Jujiang Stream (JS) | Sanchaxi Stream (SS) | Hongkouxi Stream (HKS) | |
|---|---|---|---|---|---|---|---|
| Water Environment | |||||||
| Tem (°C) | 15.30~31.22 ^ | 15.47~29.08 | 15.59~30.91 | 15.84~31.00 | 15.84~31.00 | 16.21~31.02 | |
| (22.67 ± 5.06) * | (22.39 ± 5.11) | (22.80 ± 5.34) | (23.43 ± 4.99) | (22.95 ± 4.96) | (23.90 ± 6.06) | ||
| EC (mS/cm) | 31.16~47.60 | 40.70~66.60 | 31.42~46.20 | 32.90~70.00 | 35.13~52.20 | 37.40~47.40 | |
| (42.60 ± 4.05) | (50.60 ± 4.11) | (41.01 ± 5.08) | (46.71 ± 10.37) | (43.19 ± 5.32) | (44.70 ± 4.88) | ||
| DO (mg/L) | 5.07~8.16 | 5.14~8.32 | 5.84~7.69 | 5.25~7.75 | 5.26~8.48 | 6.08~8.48 | |
| (6.78 ± 0.86) | (6.88 ± 1.06) | (6.99 ± 0.70) | (6.84 ± 0.81) | (7.02 ± 0.82) | (7.30 ± 1.00) | ||
| ORP (V) | 0.19~0.46 | 0.34~0.52 | 0.18~0.44 | 0.14~0.43 | 0.16~0.46 | 0.20~0.43 | |
| (0.37 ± 0.07) | (0.40 ± 0.05) | (0.37 ± 0.08) | (0.33 ± 0.10) | (0.35 ± 0.11) | (0.35 ± 0.10) | ||
| pH | 6.43~8.17 | 6.81~7.88 | 6.49~8.23 | 6.54~8.02 | 6.68~8.13 | 7.15~8.10 | |
| (7.21 ± 0.43) | (7.29 ± 0.30) | (7.20 ± 0.50) | (7.34 ± 0.43) | (7.36 ± 0.49) | (7.43 ± 0.45) | ||
| PCY (×103 cell/L) | 0.38~19.38 | 0.38~19.37 | 0.53~3.99 | 0.67~19.38 | 0.74~10.03 | 0.95~4.90 | |
| (2.05 ± 1.56) | (2.05 ± 1.56) | (2.02 ± 1.47) | (5.27 ± 4.74) | (3.87 ± 3.04) | (3.33 ± 1.71) | ||
| Tur (NTU) | 1.10~8.00 | 3.40~6.20 | 2.60~5.30 | 1.50~7.95 | 1.70~11.10 | 1.90~9.90 | |
| (4.34 ± 1.75) | (4.71 ± 0.89) | (4.36 ± 0.87) | (4.14 ± 1.79) | (5.13 ± 2.55) | (5.44 ± 3.62) | ||
| Chl-a (mg/L) | 0.60~4.16 | 1.24~13.66 | 0.63~7.54 | 0.50~4.79 | 1.20~6.28 | 1.05~3.58 | |
| (1.71 ± 1.11) | (4.09 ± 3.50) | (2.13 ± 2.43) | (2.31 ± 1.52) | (2.85 ± 1.61) | (2.43 ± 1.25) | ||
| TN (mg/L) | 0.10~0.63 | 0.30~0.67 | 0.2282~0.4754 | 0.14~0.47 | 0.07~0.46 | 0.17~0.49 | |
| (0.38 ± 0.12) | (0.44 ± 0.12) | (0.35 ± 0.065) | (0.33 ± 0.096) | (0.31 ± 0.14) | (0.36 ± 0.14) | ||
| CODMn (mg/L) | 0.37~2.00 | 0.72~2.30 | 0.73~1.88 | 0.75~1.80 | 0.63~1.70 | 0.78~1.89 | |
| 1.36 ± 0.43 | (1.58 ± 0.53) | (1.42 ± 0.37) | (1.42 ± 0.35) | (1.35 ± 0.43) | (1.53 ± 0.51) | ||
| TP (μg/L) | 6.10~128.40 | 4.40~40.00 | 6.10~128.40 | 6.50~36.00 | 8.30~38.80 | 10.60~30.30 | |
| (23.90 ± 23.80) | (20.90 ± 12.30) | (23.91 ± 23.82) | (22.90 ± 9.50) | (23.20 ± 11.10) | (21.30 ± 9.70) | ||
| NH3N (μg/L) | 13.10~174.30 | 7.90~235.10 | 11.80~118.60 | 10.20~155.80 | 3.20~143.00 | 3.00~170.00 | |
| (50.20 ± 42.40) | (78.30 ± 74.90) | (37.70 ± 36.30) | (52.40 ± 54.30) | (60.30 ± 46.10) | (76.20 ± 66.50) | ||
| Categories (Orders) | Name of the Species (Genus) | Frequency of Recurrence * | Degree of Dominance | Name of the Species (Genus) | Frequency of Recurrence | Degree of Dominance |
|---|---|---|---|---|---|---|
| Rotifera | Asplanchna | 100% | 2.60% | Keratella | 40% | 0.20% |
| Polyarthra | 80% | 4.31% | Testudinalla | 40% | 0.60% | |
| Trichocerca | 60% | 0.25% | Filinia | 40% | 0.53% | |
| Gastropus | 60% | 0.44% | Ascomorpha | 40% | 0.33% | |
| Mytilina | 60% | 0.49% | Eosphora | 40% | 0.63% | |
| Brachiomus | 60% | 3.07% | A.fissa | 20% | 0.02% | |
| Cephalodella | 60% | 3.72% | Rotaria | 20% | 0.26% | |
| Pompholyx | 60% | 1.16% | Epiphanes | 20% | 0.42% | |
| Synchaeta | 60% | 0.34% | Euchlanis | 20% | 0.02% | |
| Notholeca | 40% | 0.74% | Ploesoma | 20% | 0.06% | |
| Copepoda | Nitocra | 100% | 6.90% | Paracyclops | 80% | 2.58% |
| Sinocalanus | 100% | 11.80% | Mesocyclops | 80% | 1.99% | |
| Sinodiaptomus | 100% | 9.00% | Heliodiaptomus | 80% | 5.39% | |
| Cyclops | 100% | 1.51% | Onchocamptus | 60% | 0.15% | |
| Tropocyclops | 80% | 2.12% | Limnoithona | 60% | 1.06% | |
| Themocyclops | 80% | 2.23% | Neodiaptomus | 60% | 1.64% | |
| Canthocamptus | 80% | 1.47% | Macrocyclops | 40% | 0.05% | |
| Mongolodiaptpmus | 80% | 1.00% | ||||
| Cladocera | Macrothrix | 100% | 4.20% | Diaphanosoma | 60% | 5.60% |
| Daphnia | 80% | 6.12% | Camptocercus | 40% | 0.49% | |
| Bosminopsis | 60% | 1.37% | Leydigia | 20% | 0.24% | |
| Chydorus | 60% | 3.54% | Alona | 20% | 0.32% | |
| Simocephalus | 60% | 2.11% | Moinodaphnia | 20% | 0.08% | |
| Bosmina | 60% | 6.92% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Xia, J.; Xia, Z.; Cai, W.; Liu, Z.; Xu, K.; Wang, Y.; Zhang, R.; Dong, X. Temporal Variation and Spatial Distribution in the Water Environment Helps Explain Seasonal Dynamics of Zooplankton in River-Type Reservoir. Sustainability 2022, 14, 13719. https://doi.org/10.3390/su142113719
Yin J, Xia J, Xia Z, Cai W, Liu Z, Xu K, Wang Y, Zhang R, Dong X. Temporal Variation and Spatial Distribution in the Water Environment Helps Explain Seasonal Dynamics of Zooplankton in River-Type Reservoir. Sustainability. 2022; 14(21):13719. https://doi.org/10.3390/su142113719
Chicago/Turabian StyleYin, Jingyun, Jihong Xia, Zhichang Xia, Wangwei Cai, Zewen Liu, Kejun Xu, Yue Wang, Rongzhen Zhang, and Xu Dong. 2022. "Temporal Variation and Spatial Distribution in the Water Environment Helps Explain Seasonal Dynamics of Zooplankton in River-Type Reservoir" Sustainability 14, no. 21: 13719. https://doi.org/10.3390/su142113719
APA StyleYin, J., Xia, J., Xia, Z., Cai, W., Liu, Z., Xu, K., Wang, Y., Zhang, R., & Dong, X. (2022). Temporal Variation and Spatial Distribution in the Water Environment Helps Explain Seasonal Dynamics of Zooplankton in River-Type Reservoir. Sustainability, 14(21), 13719. https://doi.org/10.3390/su142113719