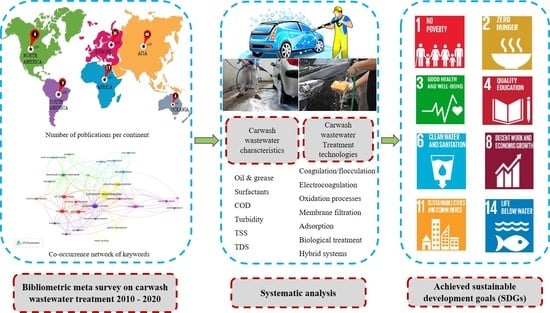
Transition towards Sustainable Carwash Wastewater Management: Trends and Enabling Technologies at Global Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Classification
2.3. Data Analysis
3. Results and Discussion
3.1. Carwash Wastewater Quantity and Composition at the Global Scale
3.2. Carwash Discharge into the Environment
3.3. Carwash Wastewater Treatment Technologies
3.3.1. Adsorption
3.3.2. Oxidation Processes
3.3.3. Electrocoagulation
3.3.4. Membrane-Based
3.3.5. Coagulation–Flocculation
3.3.6. Biological-Based Process
3.4. Performance of Carwash Wastewater Treatment Methods
3.4.1. COD Removal Efficiency
3.4.2. Oil and Grease Removal
3.4.3. Surfactant Removal Efficiency
3.4.4. Removal of Total Dissolved Solids (TDS) and Total Suspended Solids (TSS)
3.5. Optimization of Operational Factors for Enhancing Carwash Wastewater Treatment
3.6. Research Trends and Hotspots in Carwash Wastewater Management
3.7. Outlook for Achieving SDGs in Carwash Wastewater Management at Field Scale
3.7.1. Environmental-Related SDGs
3.7.2. Economic-Related SDGs
3.7.3. Social-Related SDGs
3.8. Future Perspectives in Carwash Management
- -
- Integrating the “circular economy” concept into CWW management by boosting reuse and waste minimization scenarios.
- -
- Providing appropriate analytical techniques and holistic tools to examine the cost of CWW treatment over various steps involving implementation, operation, and maintenance.
- -
- Selecting suitable CWW treatment technologies that discharge no liquid effluent into surface waters, supporting efficient recycling and reuse.
- -
- Raising environmental awareness in the population regarding the reuse of treated water in order to avoid water resource depletion and aquatic pollution.
- -
- Applying internet of things, cyber-physical systems, and machine learning towards determining optimal CWW treatment techniques.
- -
- Establishing dynamic models to optimize the performance of membrane systems and predict fouling issues due to the deposition of solids and oily particulates.
- -
- Studying pilot and large-scale systems for CWW treatment representing real processes and environmental conditions.
- -
- Encouraging the stakeholders, policymakers, and both the public and private sectors to invest in CWW treatment systems for pollution reduction and resource recovery and reuse.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallick, S.K.; Chakraborty, S. Bioremediation of wastewater from automobile service station in anoxic-aerobic sequential reactors and microbial analysis. Chem. Eng. J. 2018, 361, 982–989. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.-S. Performance of acid mine drainage sludge as an innovative catalytic oxidation source for treating vehicle-washing wastewater. J. Dispers. Sci. Technol. 2020, 43, 50–60. [Google Scholar] [CrossRef]
- Davarnejad, R.; Sarvmeili, K.; Sabzehei, M. Car Wash Wastewater Treatment Using an Advanced Oxidation Process: A Rapid Technique for the COD Reduction of Water Pollutant Sources. J. Mex. Chem. Soc. 2019, 63, 164–175. [Google Scholar] [CrossRef]
- Zaneti, R.N.; Etchepare, R.; Rubio, J. Car wash wastewater treatment and water reuse—A case study. Water Sci. Technol. 2013, 67, 82–88. [Google Scholar] [CrossRef]
- Méndez-Díaz, J.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Canonica, S.; von Gunten, U. Advanced oxidation of the surfactant SDBS by means of hydroxyl and sulphate radicals. Chem. Eng. J. 2010, 163, 300–306. [Google Scholar] [CrossRef]
- Salahi, A.; Abbasi, M.; Mohammadi, T. Permeate flux decline during UF of oily wastewater: Experimental and modeling. Desalination 2010, 251, 153–160. [Google Scholar] [CrossRef]
- Veréb, G.; Gayır, V.E.; Santos, E.N.; Fazekas, Á.; Kertész, S.; Hodúr, C.; László, Z. Purification of real car wash wastewater with complex coagulation/flocculation methods using polyaluminum chloride, polyelectrolyte, clay mineral and cationic surfactant. Water Sci. Technol. 2019, 80, 1902–1909. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Safe Use of Wastewater, Excreta and Greywater. World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Daud, Z.; Awang, H.; Latif, A.A.A.; Nasir, N.; Ridzuan, M.B.; Ahmad, Z. Suspended Solid, Color, COD and Oil and Grease Removal from Biodiesel Wastewater by Coagulation and Flocculation Processes. Procedia—Soc. Behav. Sci. 2015, 195, 2407–2411. [Google Scholar] [CrossRef] [Green Version]
- El Hanandeh, A.; Albalasmeh, A.; Gharaibeh, M.; Alajlouni, M. Modification of biochar prepared from olive oil processing waste to enhance phenol removal from synthetic and olive mill wastewater. Sep. Sci. Technol. 2020, 56, 1659–1671. [Google Scholar] [CrossRef]
- Tony, M.A.; Purcell, P.J.; Zhao, Y. Oil refinery wastewater treatment using physicochemical, Fenton and Photo-Fenton oxidation processes. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2012, 47, 435–440. [Google Scholar] [CrossRef]
- Onder, E.; Koparal, A.; Ogutveren, U. An alternative method for the removal of surfactants from water: Electrochemical coagulation. Sep. Purif. Technol. 2007, 52, 527–532. [Google Scholar] [CrossRef]
- Dimoglo, A.; Sevim-Elibol, P.; Dinç, Ö.; Gökmen, K.; Erdoğan, H. Electrocoagulation/electroflotation as a combined process for the laundry wastewater purification and reuse. J. Water Process Eng. 2019, 31, 100877. [Google Scholar] [CrossRef]
- Sanghamitra, P.; Mazumder, D.; Mukherjee, S. Treatment of wastewater containing oil and grease by biological method- a review. J. Environ. Sci. Health Part A 2021, 56, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Boluarte, I.A.; Andersen, M.; Pramanik, B.K.; Chang, C.-Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of ceramic ultrafiltration and reverse osmosis membranes in treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Mahmood, Q.; Raja, I.A.; Malik, A.H.; Khan, M.S.; Wu, D. Chemical oxidation of carwash industry wastewater as an effort to decrease water pollution. Phys. Chem. Earth 2011, 36, 465–469. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Li, C.; Champagne, P.; Anderson, B. Enhanced biogas production from anaerobic co-digestion of municipal wastewater treatment sludge and fat, oil and grease (FOG) by a modified two-stage thermophilic digester system with selected thermo-chemical pre-treatment. Renew. Energy 2015, 83, 474–482. [Google Scholar] [CrossRef]
- UN-Water. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; UN-Water: Paris, France, 2018. [Google Scholar]
- Ezz, H.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Dual biogas and biochar production from rice straw biomass: A techno-economic and sustainable development approach. Biomass-Convers. Biorefin. 2021, 1–15. [Google Scholar] [CrossRef]
- UN. Sustainable Development Goals: 17 Goals to Transform Our World. 2015. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 20 August 2021).
- Durán-Sánchez, A.; Álvarez-García, J.; González-Vázquez, E.; Río-Rama, M.D.L.C.D.D. Wastewater Management: Bibliometric Analysis of Scientific Literature. Water 2020, 12, 2963. [Google Scholar] [CrossRef]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 18th ed.; Port City Press: Baltimore, MD, USA, 1992. [Google Scholar]
- Ranjbari, M.; Saidani, M.; Esfandabadi, Z.S.; Peng, W.; Lam, S.S.; Aghbashlo, M.; Quatraro, F.; Tabatabaei, M. Two decades of research on waste management in the circular economy: Insights from bibliometric, text mining, and content analyses. J. Clean. Prod. 2021, 314, 128009. [Google Scholar] [CrossRef]
- Tajuddin, M.F.; Al-Gheethi, A.; Mohamed, R.; Noman, E.; Talip, B.A.; Bakar, A. Optimizing of heavy metals removal from car wash wastewater by chitosan-ceramic beads using response surface methodology. Mater. Today Proc. 2020, 31, 43–47. [Google Scholar] [CrossRef]
- Monney, I.; Donkor, E.A.; Buamah, R. Clean vehicles, polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 2020, 6, e03952. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Moazzem, S.; Jegatheesan, V. Treating Car Wash Wastewater by Ceramic Ultrafiltration Membranes for Reuse Purposes. In Water Scarcity and Ways to Reduce the Impact; Springer: Berlin/Heidelberg, Germany, 2018; pp. 63–73. [Google Scholar] [CrossRef]
- Yılmaz Nayır, T.; Kara, S. Container washing wastewater treatment by combined electrocoagulation–electrooxidation. Sep. Sci. Technol. 2018, 53, 1592–1603. [Google Scholar] [CrossRef]
- Rubi-Juarez, H.; Barrera-Diaz, C.; Urena-Nunez, F. Adsorption-assisted electrocoagulation of real car wash wastewater with equilibrium and kinetic studies. Pollut. Res. 2017, 36, 175–184. [Google Scholar]
- Ganiyu, S.O.; dos Santos, E.V.; Costa, E.C.T.D.A.; Martínez-Huitle, C.A. Electrochemical advanced oxidation processes (EAOPs) as alternative treatment techniques for carwash wastewater reclamation. Chemosphere 2018, 211, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Kumi, A.G.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Petrochemical Wastewater Treatment by Eggshell Modified Biochar as Adsorbent: Atechno-Economic and Sustainable Approach. Adsorpt. Sci. Technol. 2022, 2022, 2323836. [Google Scholar] [CrossRef]
- Rosli, M.A.; Sa’ari, S.N.; Rahman MA, A.; Rahman MF, A.; Sunar, N.M.; Awang, M.; Najib MZ, M. The effectiveness of macrocomposite adsorbent for treatment of COD and suspended solid of car wash water effluent. Int. J. Emerg. Trends Eng. Res. 2020, 8, 34–39. [Google Scholar] [CrossRef]
- Veit, M.T.; Novais, G.V.; Juchen, P.T.; Palácio, S.M.; Gonçalves, G.D.C.; Zanette, J.C. Automotive Wash Effluent Treatment Using Combined Process of Coagulation/Flocculation/Sedimentation–Adsorption. Water Air Soil Pollut. 2020, 231, 1–12. [Google Scholar] [CrossRef]
- Karci, A.; Arslan-Alaton, I.; Bekbolet, M. Advanced oxidation of a commercially important nonionic surfactant: Investigation of degradation products and toxicity. J. Hazard. Mater. 2013, 263, 275–282. [Google Scholar] [CrossRef]
- Alabdly, H.A.; Jaafar, M.S.; Kamil, S.A.; Alabdly, H.A.; Jaafar, M.S. A comparative Study for Grey Water Using Ozonation and Ultrasonic Irradiation Processes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 022095. [Google Scholar]
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef] [PubMed]
- El Shahawy, A.; Ahmed, I.A.; Nasr, M.; Ragab, A.H.; Al-Mhyawi, S.R.; Elamin, K.M.A. Organic Pollutants Removal from Olive Mill Wastewater Using Electrocoagulation Process via Central Composite Design (CCD). Water 2021, 13, 3522. [Google Scholar] [CrossRef]
- Jiku, Z.; Yanbin, Y.; Huiye, W.; Zhibiao, D. CFU combined process for the treatment of oily car washing wastewater. Appl. Mech. Mater. 2013, 253–255, 999–1004. [Google Scholar] [CrossRef]
- Kumi, A.G.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Synthesis of sludge-derived biochar modified with eggshell waste for monoethylene glycol removal from aqueous solutions. SN Appl. Sci. 2020, 2, 1696. [Google Scholar] [CrossRef]
- Istirokhatun, T.; Destianti, P.; Hargianintya, A.; Oktiawan, W.; Susanto, H. Treatment of car wash wastewater by UF membranes. AIP Conf. Proc. 2015, 1699, 060025. [Google Scholar] [CrossRef]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ. Sci. Pollut. Res. 2015, 23, 3195–3216. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Miino, M.C.; Baldi, M.; Manzi, S.; Abbà, A.; Bertanza, G. Removal of non-ionic and anionic surfactants from real laundry wastewater by means of a full-scale treatment system. Process Saf. Environ. Prot. 2019, 132, 105–115. [Google Scholar] [CrossRef]
- Monney, I.; Buamah, R.; Donkor, E.A.; Etuaful, R.; Nota, H.K.; Ijzer, H. Treating waste with waste: The potential of synthesized alum from bauxite waste for treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2019, 26, 12755–12764. [Google Scholar] [CrossRef]
- Magnago, R.F.; Berselli, D.; Medeiros, P. Treatment of wastewater from car wash by fenton and photo-fenton oxidative processes. J. Eng. Sci. Technol. 2018, 13, 838–850. [Google Scholar]
- Lau, W.J.; Ismail, A.F.; Firdaus, S. Car wash industry in Malaysia: Treatment of car wash effluent using ultrafiltration and nanofiltration membranes. Sep. Purif. Technol. 2013, 104, 26–31. [Google Scholar] [CrossRef]
- Maqbool, F.; Kamal, R.; Bhatti, Z.A.; Pervez, S.; Sajid, M.; Haleem, K.; Faridullah, F. Effects of hydrocarbon degrading inoculum for carwash effluent treatment in a UASB reactor. Desalination Water Treat. 2019, 164, 31–38. [Google Scholar] [CrossRef]
- Moazzem, S.; Ravishankar, H.; Fan, L.; Roddick, F.; Jegatheesan, V. Application of enhanced membrane bioreactor (eMBR) for the reuse of carwash wastewater. J. Environ. Manag. 2019, 254, 109780. [Google Scholar] [CrossRef] [PubMed]
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. An integrated electrocoagulation–nanofiltration process for carwash wastewater reuse. Chemosphere 2020, 253, 126713. [Google Scholar] [CrossRef]






| Asia | Africa | Europe | North America | South America | Oceania | |
|---|---|---|---|---|---|---|
| Volume (L/vehicle) | 462–758 (n = 9) | 150–347 (n = 3) | 275–425 (n = 7) | 97–171 (n = 2) | 310–450 (n = 4) | 145–200 (n = 3) |
| pH | 6.4–13.8 (n = 39) | 6.9–8.6 (n = 26) | 5.5–11.5 (n = 17) | 6.3–7.8 (n = 3) | 4.4–7.7 (n = 11) | 6.1–8.5 (n = 6) |
| COD (mg/L) | 1418–2032 (n = 51) | 936–1413 (n = 11) | 9716–14010 (n = 16) | 682–1024 (n = 3) | 459–683 (n = 13) | 580–944 (n = 6) |
| Oil and grease (mg/L) | 860–1395 (n = 34) | 10–50 (n = 18) | 76–125 (n = 4) | 300–448 (n = 5) | 71–112 (n = 8) | 25–83 (n = 3) |
| Surfactants (mg/L) | 145–189 (n = 12) | 2–9 (n = 7) | 214–290 (n = 10) | 3–9 (n = 4) | 77–119 (n = 8) | 82–106 (n = 2) |
| Turbidity (NTU) | 786–1194 (n = 32) | 2810–4000 (n = 24) | 212–366 (n = 8) | 547–925 (n = 5) | 242–426 (n = 11) | 689–1000 (n = 5) |
| TSS (mg/L) | 940–1750 (n = 26) | 2055–3417 (n = 5) | 1450–2300 (n = 9) | 319–538 (n = 7) | 68–260 (n = 7) | 807–1275 (n = 5) |
| TDS (mg/L) | 5521–7920 (n = 26) | 448–686 (n = 20) | 674–1054 (n = 2) | 11876–17268 (n = 4) | 520–803 (n = 9) | 527–818.4 (n = 4) |
| CWW Treatment Method | Operational Factors | Removal Efficiency (%) | Reference | |||
|---|---|---|---|---|---|---|
| COD | Oil and Grease | Surfactants | TDS/TSS | |||
| Coagulation/flocculation by Poly-Aluminium Chloride (PACl) | 100 mg/L Na-bentonite, 20 mg/L Al3+, and 0.5 mg/L anionic polyelectrolyte | 59.0 | 85.0 | – | – | [7] |
| Coagulation/flocculation by alum | 12.5 mL 10% Alum, and 10 mL 5% PACl per 1 L CWW | 67.4 | – | – | 97.9 (TSS) | [15] |
| Coagulation/flocculation by synthesized alum from bauxite waste | 90 mg/L alum, 200 rpm@2 min, 25 rpm@5 min, and 34 min sedimentation | 75.0 | – | 34.0 | – | [44] |
| Oxidation by electro-Fenton (EF) | 75.8 min, 58.8 mA/cm2, pH 3.02, 1.62 mL/L H2O2/CWW, and 3.66 H2O2/Fe2+ | 68.7 | – | 73.6 | 71.8 (TSS) | [3] |
| Oxidation by electrooxidation with H2O2 generation | 0.5 mM Fe2+, pH 3, and 500 mA | 96.0 | 96.0 | – | – | [31] |
| Oxidation by Fenton | pH 3.0, and 1 h | 83.3–83.9 | 88.5–89.0 | 94.1–95.2 | – | [45] |
| Oxidation by photo-Fenton | two 40 W UVA radiation, pH 3.0, and 1 h | 92.3–93.9 | 98.9–99.6 | 100 | – | [45] |
| Adsorption by macro-composite | 10 mL/min flowrate, 38.2 min, and 0.1 cm/min surface loading rate | 88.0 | – | – | 92.3 (TSS) | [33] |
| Membrane ultra-filtration | 1 bar pressure, and 2.69 L/m2/h | 95.0 | 100 | – | – | [41] |
| Membrane nano-filtration | 3 bar pressure, and 58.5 L/m2/h | 70.9–91.5 | – | – | 60.0–61.5 (TDS) | [46] |
| Membrane ultra-filtration | 3 bar pressure, and 58.5 L/m2/h | 54.9–83.9 | – | – | 17.6–31.5 (TDS) | [46] |
| Biological up-flow anaerobic sludge blanket (UASB) reactor | 4 d hydraulic retention time | 96.0 | 96.8 | – | 11.0 (TDS) | [47] |
| Biological anoxic - aerobic sequential reactor | 24 h hydraulic retention time | 94.0 | – | – | – | [1] |
| Hybrid coagulation/flocculation + Adsorption | 220 mg/L coagulant, and 2 h sorption | 92.6 | – | 97.2 | 35.6 (TDS) | [34] |
| Hybrid bioreactor + UV Lamp + membrane filtration | 6.6 J/cm2 UV dosage, 10.13 L/m2/h flux, 50.8 kPa, and 94 h | 99.9 | 80.0 | 99.9 | 100 (TSS); 25.6 (TDS) | [48] |
| Hybrid flocculation-column flotation + sand filtration + chlorination | 0.5 mg Cl2/L | 62.8 | 27.3 | 42.9 | 91.0 (TSS) | [4] |
| Hybrid aeration + coagulation/flocculation + oxidation | 90 min aeration, 80 mg/L alum, and 2.5 mL/L waste H2O2 | 93.0 | 96.3 | – | 14.0 (TDS) | [17] |
| Hybrid coagulation/flocculation + sand filtration + ceramic ultrafiltration (UF) + reverse osmosis (RO) | 45 mg/L FeCl3 coagulant, 250 kPa UF, and1000 kPa RO | 96.0 | – | – | 100 (TSS); 42.5 (TDS) | [16] |
| Hybrid electrocoagulation + electrooxidation | Fe electrodes, 25 mA/cm2, pH 5, and 120 min | 90.2 | – | – | 80.7 (TSS) | [29] |
| Hybrid electrocoagulation + adsorption | Al electrodes, and 22.5 g/L coagulant | 99.1 | 100 | – | 95.9 (TSS) | [30] |
| Hybrid coagulation + flotation + ultrafiltration | 150 mg/L coagulant, 0.2 m³/h, 1:9 gas-liquid ratio, and 0.3–0.4 MPa | – | 40.0 | – | – | [39] |
| Hybrid electrocoagulation and nanofiltration | 25 °C, 250 rpm, and parallel connection of monopolar electrodes | 88.0 | 90.0 | 91.0 | 99.0 (TSS) | [49] |
| Hybrid coagulation/flocculation followed by sedimentation + sand filtration + ceramic membrane filtration | 300 rpm @ 1 min (coagulation), 30 rpm @ 20 min, 30 min sedimentation, 3.5 m/h in filter, and 2 bar in membrane | 78.3–79.8 | – | – | 14.5 (TDS); 100 (TSS) | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadebo, D.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Transition towards Sustainable Carwash Wastewater Management: Trends and Enabling Technologies at Global Scale. Sustainability 2022, 14, 5652. https://doi.org/10.3390/su14095652
Dadebo D, Ibrahim MG, Fujii M, Nasr M. Transition towards Sustainable Carwash Wastewater Management: Trends and Enabling Technologies at Global Scale. Sustainability. 2022; 14(9):5652. https://doi.org/10.3390/su14095652
Chicago/Turabian StyleDadebo, Derrick, Mona G. Ibrahim, Manabu Fujii, and Mahmoud Nasr. 2022. "Transition towards Sustainable Carwash Wastewater Management: Trends and Enabling Technologies at Global Scale" Sustainability 14, no. 9: 5652. https://doi.org/10.3390/su14095652
APA StyleDadebo, D., Ibrahim, M. G., Fujii, M., & Nasr, M. (2022). Transition towards Sustainable Carwash Wastewater Management: Trends and Enabling Technologies at Global Scale. Sustainability, 14(9), 5652. https://doi.org/10.3390/su14095652