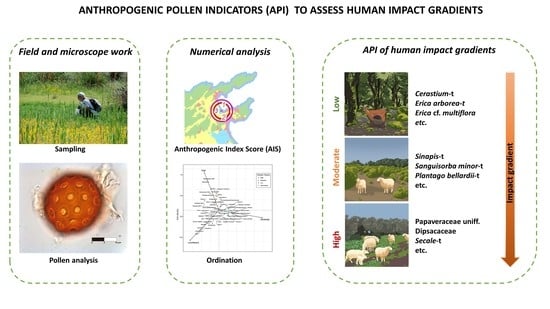
Human-Impact Gradients through Anthropogenic Pollen Indicators in a Mediterranean Mosaic Landscape (Balearic Islands)
Abstract
:1. Introduction
2. Study Area
3. Materials and Methods
3.1. Fieldwork
3.2. Environmental Variables
3.3. Pollen Analysis
3.4. Statistical Analysis
3.4.1. Data Preparation and Statistical Software
3.4.2. Redundancy Analysis (RDA) of Pollen Taxa and Environmental Variables
3.4.3. Constructing the Anthropogenic Intensity Score (AIS) for Sample Sites
- (1)
- At each sample site, the values of the 8 most influential environmental variables were compiled and transformed to equalize the magnitude of each measure. For example, agropastoral use is a binary variable with values ranging from 0 to 1, while herbivory pressure is an ordinal variable with values ranging from 0 to 2. To give both variables equal magnitude in constructing the AIS, the values of the agropastoral use variable are multiplied by 2 to match the range of values of the herbivory pressure variable. This logic was applied to all variables in our dataset.
- (2)
- The resulting values of each environmental variable were weighted by the p-values calculated by the ANOVA permutation tests applied to the results of the RDA, following Equation (1). Weighting these variables gave the more influential environmental predictors greater effect in determining the AIS than less influential environmental variables.
- (3)
- Weighted environmental variables were then summed for each sample site to create a raw anthropogenic intensity score value.
- (4)
- The final AIS was calculated by rescaling the raw anthropogenic intensity values for each site to a 0 to 1 index, with 1 representing the highest anthropogenic intensity score and 0 the lowest anthropogenic intensity score.
3.4.4. Evaluating Anthropogenic Indicator Gradients
4. Results
4.1. Pollen Type-Environmental Variables Correlation
4.2. Redundancy Analysis (RDA)
4.3. K-means Clustering and Unconstrained PCA with Anthropogenic Impact Gradient Categories
5. Discussion
5.1. Identifying Local/Microregional and Regional Anthropogenic Pollen Indicators
5.2. Pollen Types Related to Different Degrees of Human Impact Intensity
5.3. Reflection on Pollen Morphology Resolution and Productive Cultural Practices
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaillard, M.J. Pollen Methods and Studies: Archaeological Applications. In Encyclopedia of Quaternary Sciences; Elias, S.A., Mock, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3, pp. 880–9903. [Google Scholar]
- Behre, K.-E. The Interpretation of Anthropogenic Indicators in Pollen Diagrams. Pollen Et Spores 1981, 23, 225–245. [Google Scholar]
- Gauthier, E. Pollen et Microfossiles: Des Crêts Du Jura Aux Fjords Du Groenland; Habilitation à Diriger des Recherches, Université de Franche-Comté: Besançon, France, 2012. [Google Scholar]
- Mazier, F.; Galop, D.; Brun, C.; Buttler, A. Modern Pollen Assemblages from Grazed Vegetation in the Western Pyrenees, France: A Numerical Tool for More Precise Reconstruction of Past Cultural Landscapes. Holocene 2006, 16, 91–103. [Google Scholar] [CrossRef]
- Brun, C.; Dessaint, F.; Richard, H.; Bretagnolle, F. Arable-Weed Flora and Its Pollen Representation: A Case Study from the Eastern Part of France. Rev. Palaeobot. Palynol. 2007, 146, 29–50. [Google Scholar] [CrossRef]
- Ejarque, A.; Miras, Y.; Riera, S. Pollen and Non-Pollen Palynomorph Indicators of Vegetation and Highland Grazing Activities Obtained from Modern Surface and Dung Datasets in the Eastern Pyrenees. Rev. Palaeobot. Palynol. 2011, 167, 123–139. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Bandini, M.M.; Florenzano, A.; Montecchi, M.C.; Rattighieri, E.; Torri, P. Anthropogenic Pollen Indicators (API) from Archaeological Sites as Local Evidence of Human-Induced Environments in the Italian Peninsula. Ann. Di Bot. 2013, 3, 143–153. [Google Scholar] [CrossRef]
- Deza-Araujo, M.; Morales-Molino, C.; Conedera, M.; Henne, P.D.; Krebs, P.; Hinz, M.; Heitz, C.; Hafner, A.; Tinner, W. A New Indicator Approach to Reconstruct Agricultural Land Use in Europe from Sedimentary Pollen Assemblages. Palaeogeogr. Palaeoclim. Palaeoecol. 2022, 599, 111051. [Google Scholar] [CrossRef]
- Deza-Araujo, M.; Morales-Molino, C.; Tinner, W.; Henne, P.D.; Heitz, C.; Pezzatti, G.B.; Hafner, A.; Conedera, M. A Critical Assessment of Human-Impact Indices Based on Anthropogenic Pollen Indicators. Quat. Sci. Rev. 2020, 236, 106291. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Blondel, J. On Humans and Wildlife in Mediterranean Islands. J. Biogeogr. 2008, 35, 509–518. [Google Scholar] [CrossRef]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe. An Ecological History; Yale University Press: New Haven, CT, USA; London, UK, 2001. [Google Scholar]
- Woodbridge, J.; Roberts, N.; Fyfe, R. Pan-Mediterranean Holocene Vegetation and Land-Cover Dynamics from Synthesized Pollen Data. J. Biogeogr. 2018, 45, 2159–2174. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Florenzano, A.; Burjachs, F.; Giardini, M.; Kouli, K.; Masi, A.; Picornell-Gelabert, L.; Revelles, J.; Sadori, L.; Servera-Vives, G.; et al. From Influence to Impact: The Multifunctional Land Use in Mediterranean Prehistory Emerging from Palynology of Archaeological Sites (8.0-2.8 Ka BP). Holocene 2019, 29, 830–846. [Google Scholar] [CrossRef]
- Murray, I.; Jover-Avellà, G.; Fullana, O.; Tello, E. Biocultural Heritages in Mallorca: Explaining the Resilience of Peasant Landscapes within a Mediterranean Tourist Hotspot, 1870–2016. Sustainability 2019, 11, 1926. [Google Scholar] [CrossRef]
- Dawson, H. Island Archaeology. In Encyclopedia of Global Archaeology; Springer: Cham, Switzerland, 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Nogué, S.; Santos, A.M.C.; John, H.; Björck, S.; Castilla-Beltrán, A.; Connor, S.; de Boer, E.J.; de Nascimento, L.; Felde, V.A.; Fernández-Palacios, J.M.; et al. The Human Dimension of Biodiversity Changes on Islands. Science 2021, 372, 488–491. [Google Scholar] [CrossRef]
- Servera-Vives, G.; Mus Amezquita, M.; Snitker, G.; Florenzano, A.; Torri, P.; Estrany Bertos, J.; Mercuri, A.M. Modern Analogs for Understanding Pollen-Vegetation Dynamics in a Mediterranean Mosaic Landscape (Balearic Islands, Western Mediterranean). Holocene 2022, 32, 716–734. [Google Scholar] [CrossRef]
- Morey, M.; Ruiz-Pérez, M. The Balearic Islands. In Mediterranean Island Landscapes: Natural and Cultural Approaches; Vogiatzakis, I., Pungetti, G., Mannion, A.M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 271–296. ISBN 978-1-4020-5064-0. [Google Scholar]
- Guijarro, J.A. Contribución a La Bioclimatología de Baleares; Universitat de les Illes Balears: Palma, Spain, 1986. [Google Scholar]
- Picornell-Gelabert, L.; Servera-Vives, G. Landscape Practices and Everyday Life in Domestic Spaces in Bronze Age Mallorca (Balearic Islands): Perspectives for and Archaeology of Fuel and Firewood. Quat. Int. 2017, 431, 73–89. [Google Scholar] [CrossRef]
- Servera-Vives, G.; Riera, S.; Picornell-Gelabert, L.; Moffa-Sánchez, P.; Llergo, Y.; Garcia, A.; Mus-Amezquita, M.; García Álvarez, S.; Calvo Trías, M. The Onset of Islandscapes in the Balearic Islands: A Study-Case of Addaia (Northern Minorca, Spain). Palaeogeogr. Palaeoclim. Palaeoecol. 2018, 498, 9–23. [Google Scholar] [CrossRef]
- Llorens, L.; Gil, L. The Balearic Islands. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Series “Plant and Vegetation”; Springer: Cham, Switzerland, 2017; Volume 2, pp. 3–33. ISBN 978-3-319-54866-1. [Google Scholar]
- Pons Esteva, A.; Rullán Salamanca, O. Artificialization and Islandness on the Spanish Tourist Coast. Misc. Geogr. 2014, 18, 5–16. [Google Scholar] [CrossRef]
- Capó, M.; Roig-Oliver, M.; Cardona, C.; Cursach, J.; Bartolomé, J.; Rita, J.; Baraza, E. Historic Exposure to Herbivores, Not Constitutive Traits, Explains Plant Tolerance to Herbivory in the Case of Two Medicago Species (Fabaceae). Plant Sci. 2021, 307, 110890. [Google Scholar] [CrossRef]
- Dante Cerrato, M.; Ribas-Serra, A.; Miquel Mir-Rosselló, P.; Cardona Ametller, C.; Gil-Vives, L. Time Pattern Variation of Alien Plant Introductions in an Insular Biodiversity Hotspot: The Balearic Islands as a Case Study for the Mediterranean Region. Biodivers. Conserv. 2023. [Google Scholar] [CrossRef]
- Podda, L.; Fraga, I.; Arguimbau, P.; Mascia, F.; Mayoral García-Berlanga, O.; Bacchetta, G. Comparison of the Invasive Alien Flora in Continental Islands: Sardinia (Italy) and Balearic Islands (Spain). Rend. Lincei 2011, 22, 31–45. [Google Scholar] [CrossRef]
- Moragues, E.; Rita, J. Espècies Introduïdes Balears. In Conselleria de Medi Ambient; Govern de les Illes Balears: Palma, Spain, 2005; Volume 11. [Google Scholar]
- Räsänen, S. Tracing and Interpreting Fine-Scale Human Impact in Northern Fennoscandia with the Aid of Modern Pollen Analogues. Veg. Hist. Archaeobotany 2001, 10, 211–218. [Google Scholar] [CrossRef]
- Räsänen, S.; Suutari, H.; Nielsen, A.B. A Step Further towards Quantitative Reconstruction of Past Vegetation in Fennoscandian Boreal Forests: Pollen Productivity Estimates for Six Dominant Taxa. Rev. Palaeobot. Palynol. 2007, 146, 208–220. [Google Scholar] [CrossRef]
- Florenzano, A.; Mercuri, A.M.; Rinaldi, R.; Rattighieri, E.; Fornaciari, R.; Messora, R.; Arru, L. The Representativeness of Olea Pollen Fromolive Groves and the Late Holocene Landscape Reconstruction in Central Mediterranean. Front. Earth Sci. 2017, 5, 85. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Fitosociologia Bases Para El Estudio de Las Comunidades Vegetales; Blume: Madrid, Spain, 1979; ISBN 8472141748/9788472141742. [Google Scholar]
- Erdtman, G. The Acetolysis Method—A Revised Description. Vensk. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Faegri, K.; Kaland, P.E.; Krzywinski, K. Textbook of Pollen Analysis, 4th ed.; The Blackburn Press: Caldwell, NJ, USA, 1989; ISBN 978-1930665019. [Google Scholar]
- Beug, H.J. Leitfaden der Pollenbestimmung Für Mitteleuropa und Angrenzende Gebiete; Verlag Dr. Friedrich Pfeil: München, Germany, 2004; ISBN 3-89937-043-0. [Google Scholar]
- Reille, M. Pollen et Spores d’Europe et d’Afrique Du Nord. In Atlas Photographique; Laboratoire de Botanique Historique et Palynologie, URA 1152/CNRS: Marseille, France, 1992. [Google Scholar]
- Chester, P.I.P.I.; Raine, J.I.; Raine, I. Pollen and Spore Keys for Quaternary Deposits in the Northern Pindos Mountains, Greece. Grana 2001, 40, 299–387. [Google Scholar] [CrossRef]
- Punt, W.; Hoen, P.P. The Northwest European Pollen Flora, 70. Asteraceae—Asteroideae. Rev. Palaeobot. Palynol. 2009, 157, 22–183. [Google Scholar] [CrossRef]
- Tweddle, J.C.; Edwards, K.J.; Fieller, N.R.J.J. Multivariate Statistical and Other Approaches for the Separation of Cereal from Wild Poaceae Pollen Using a Large Holocene Dataset. Veg. Hist. Archaeobot. 2005, 14, 15–30. [Google Scholar] [CrossRef]
- Kouli, K. Plant Landscape and Land Use at the Neolithic Lake Settlement of Dispilió (Macedonia, Northern Greece). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2015, 149, 195–204. [Google Scholar] [CrossRef]
- Florenzano, A.; Marignani, M.; Rosati, L.; Fascetti, S.; Mercuri, A.M. Are Cichorieae an Indicator of Open Habitats and Pastoralism in Current and Past Vegetation Studies? Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2015, 149, 154–165. [Google Scholar] [CrossRef]
- López-Sáez, J.A.; Glais, A.; Tsiftsis, S.; Lezpez, L. Modern Pollen–Vegetation Relationships along an Altitudinal Transect in the Lefka Ori Massif (Western Crete, Greece). Rev. Palaeobot. Palynol. 2018, 259, 159–170. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.2.0; R Foundation for Statistical Computing; R Core Team: Viena, Austria, 2022.
- Oksanen, J.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. Vegan: Community Ecology Package. R Package, version 2.6-4; Community Ecology Package; DataCamp: New York, NY, USA, 2020.
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 9783319242750. [Google Scholar]
- Smilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139627061. [Google Scholar]
- López-Sáez, J.A.; Camarero, J.J.; Abel-Schaad, D.; Luelmo-Lautenschlaeger, R.; Pérez-Díaz, S.; Alba-Sánchez, F.; Carrión, J.S. Don’t Lose Sight of the Forest for the Trees! Discerning Iberian Pine Communities by Means of Pollen-Vegetation Relationships. Rev. Palaeobot. Palynol. 2020, 281, 104285. [Google Scholar] [CrossRef]
- López Sáez, J.; Burjachs i Casas, F.; López García, P.; Sáez, L.; García, L. Arqueopalinología: Síntesis Crítica. Polen 2003, 35, 5–35. [Google Scholar]
- Davis, O.K. Pollen Frequencies Reflect Vegetation Patterns in a Great Basin (USA) Mountain Range. Rev. Palaeobot. Palynol. 1984, 40, 295–315. [Google Scholar] [CrossRef]
- Fall, P.L. Modern Vegetation, Pollen and Climate Relationships on the Mediterranean Island of Cyprus. Rev. Palaeobot. Palynol. 2012, 185, 79–92. [Google Scholar] [CrossRef]
- McGlone, M.S.; Meurk, C.D. Modern Pollen Rain, Subantarctic Campbell Island, New Zealand. N. Z. J. Ecol. 2000, 24, 181–194. [Google Scholar]
- Ubera, J.L.; Galán, C.; Guerrero, F.H. Palynological Study of the Genus Plantago in the Iberian Peninsula. Grana 1988, 27, 1–15. [Google Scholar] [CrossRef]
- Llorens, L.; Gil, L.; Tébar, F.J. La Vegetació de l’illa de Mallorca. Bases per a La Definició i Gestió Dels Hàbitats. In Conselleria de Medi Ambient; Govern de les Illes Balears: Palma, Spain, 2007; ISBN 978-84-612-0488-5. [Google Scholar]
- Vicente, O.; Boscaiu, M.; Naranjo, M.A.Á.; Estrelles, E.; Bellés, J.M.M.; Soriano, P. Responses to Salt Stress in the Halophyte “Plantago crassifolia” (Plantaginaceae). J. Arid. Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Cañellas-Boltà, N.; Rull, V.; Vigo, J.; Mercadé, A. Modern Pollen-Vegetation Relationships along an Altitudinal Transect in the Central Pyrenees (Southwestern Europe). Holocene 2009, 19, 1185–1200. [Google Scholar] [CrossRef]
- Court-Picon, M.; Buttler, A.; De Beaulieu, J.L. Modern Pollen-Vegetation Relationships in the Champsaur Valley (French Alps) and Their Potential in the Interpretation of Fossil Pollen Records of Past Cultural Landscapes. Rev. Palaeobot. Palynol. 2005, 135, 13–39. [Google Scholar] [CrossRef]
- Beffa, G.; Pedrotta, T.; Colombaroli, D.; Henne, P.D.; van Leeuwen, J.F.N.; Süsstrunk, P.; Kaltenrieder, P.; Adolf, C.; Vogel, H.; Pasta, S.; et al. Vegetation and Fire History of Coastal North-Eastern Sardinia (Italy) under Changing Holocene Climates and Land Use. Veg. Hist. Archaeobot. 2016, 25, 271–289. [Google Scholar] [CrossRef]
- Pedrotta, T.; Gobet, E.; Schwörer, C.; Beffa, G.; Butz, C.; Henne, P.D.; Morales-Molino, C.; Pasta, S.; van Leeuwen, J.F.N.; Vogel, H.; et al. 8000 Years of Climate, Vegetation, Fire and Land-Use Dynamics in the Thermo-Mediterranean Vegetation Belt of Northern Sardinia (Italy). Veg. Hist. Archaeobot. 2021, 30, 789–813. [Google Scholar] [CrossRef] [PubMed]
- Paula, S.; Naulin, P.I.; Arce, C.; Galaz, C.; Pausas, J.G. Lignotubers in Mediterranean Basin Plants. Plant Ecol. 2016, 217, 661–676. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-da-Glória, B.; Fidelis, A. Unearthing Belowground Bud Banks in Fire-Prone Ecosystems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef]
- Paula, S.; Ojeda, F. Resistance of Three Co-Occurring Resprouter Erica Species to Highly Frequent Disturbance. Plant Ecol. 2006, 183, 329–336. [Google Scholar] [CrossRef]
- Court-Picon, M.; Buttler, A.; De Beaulieu, J.L. Modern Pollen/Vegetation/Land-Use Relationships in Mountain Environments: An Example from the Champsaur Valley (French Alps). Veg. Hist. Archaeobot. 2006, 15, 151–168. [Google Scholar] [CrossRef]
- Hjelle, K.L. Modem Pollen Assemblages from Mown and Grazed Vegetation Types in Western Norway. Rev. Palaeobot. Palynol. 1999, 107, 55–81. [Google Scholar] [CrossRef]
- Marinova, E.; Atanassova, J. Anthropogenic Impact on Vegetation and Environment during the Bronze Age in the Area of Lake Durankulak, NE Bulgaria: Pollen, Microscopic Charcoal, Non-Pollen Palynomorphs and Plant Macrofossils. Rev. Palaeobot. Palynol. 2006, 141, 165–178. [Google Scholar] [CrossRef]
- Behre, K.-E. The rôle of man in European vegetation history. In Vegetation History. Handbook of Vegetation Science; Huntley, B., Webb, T., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 7. [Google Scholar] [CrossRef]
- Riera Mora, S.; Servera-Vives, G.; Picornell-Gelabert, L.; Cabanis, M.; Boi, M.; Miras, Y. Pollen Signatures of a Ritual Process in the Collective Burial Cave of Cova des Pas (Late Bronze Age, Minorca, Balearic Islands, Spain). In The Bioarchaeology of Ritual and Religion; Livarda, A., Madgwick, R., Santiago, R., Eds.; Oxbow: Oxford, UK, 2018; pp. 28–43. ISBN 978-1-78570-828-2. [Google Scholar]
- Sadori, L.; Allevato, E.; Bellini, C.; Bertacchi, A.; Boetto, G.; Di Pasquale, G.; Giachi, G.; Giardini, M.; Masi, A.; Pepe, C.; et al. Archaeobotany in Italian Ancient Roman Harbours. Rev. Palaeobot. Palynol. 2015, 218, 217–230. [Google Scholar] [CrossRef]
- Sadori, L.; Giardini, M.; Giraudi, C.; Mazzini, I. The Plant Landscape of the Imperial Harbour of Rome. J. Archaeol. Sci. 2010, 37, 3294–3305. [Google Scholar] [CrossRef]
- Russo Ermolli, E.; Romano, P.; Ruello, M.R.; Barone Lumaga, M.R. The Natural and Cultural Landscape of Naples (Southern Italy) during the Graeco-Roman and Late Antique Periods. J. Archaeol. Sci. 2014, 42, 399–411. [Google Scholar] [CrossRef]
- Montecchi, M.C.; Mercuri, A.M. When Palynology Meets Classical Archaeology: The Roman and Medieval Landscapes at the Villa Del Casale Di Piazza Armerina, UNESCO Site in Sicily. Archaeol. Anthropol. Sci. 2016, 10, 743–757. [Google Scholar] [CrossRef]
- Bandini Mazzanti, M.; Bosi, G.; Mercuri, A.M.; Accorsi, C.A.; Guarnieri, C. Plant Use in a City in Northern Italy during the Late Medieval and Renaissance Periods: Results of the Archaeobotanical Investigation of “The Mirror Pit” (14th–15th Century AD) in Ferrara. Veg. Hist. Archaeobotany 2005, 14, 442–452. [Google Scholar] [CrossRef]






| Regional Indicators | Local/Microregional Indicators | |
|---|---|---|
| Distance to forest | Plantago cf. crassifolia, Verbascum-t, Hornungia-t, Plantago coronopus-t, Lotus-t, Apiaceae, Plantago bellardii-t, Centaurea jacea-t, Linaria-t, Chenopodiaceae, Brassicaceae SUM | Cistus salviifolius, Artemisia |
| Pasture area | Papaveraceae undiff, Dipsacaceae, Vitis, Trifolium-t, Plantago sp., Cirsium-t, Senecio-t, Cichorieae, Brassicaceae SUM, Chenopodiaceae, Rumex, Carduus-t, Apiaceae, Sinapis-t, Lotus-t, Plantago albicans, Plantago coronopus-t, Hornungia-t, Plantago major/media, Echium, Centaurea | Poaceae undiff., Polygonum aviculare-t, Cerealia SUM, Matricaria-t, Urtica dioica-t |
| Landscape openness | Trifolium-t, Vitis, Papaveraceae undiff, Dipsacaceae, Plantago sp., Cirsium-t, Senecio-t, Brassicaceae SUM, Chenopodiaceae, Cichorieae, Apiaceae, Lotus-t, Rumex, Plantago coronopus-t, Carduus-t, Sinapis-t, Hornungia-t, Plantago albicans | Poaceae undiff., Polygonum aviculare-t, Cerealia SUM, Matricaria-t |
| Agropastoral use | Plantago lanceolata-t, Rubus, Galium-t, Caryophyllaceae undiff., Centaurea, Echium, Plantago major/media | Avena/Triticum-group |
| List of potential local/microregional indicators of anthropized habitats | Poaceae undiff., Polygonum aviculare-t, Avena/Triticum-group, Cerealia SUM, Matricaria-t, Urtica dioica-t | |
| List of potential regional indicators of anthropized habitats | Plantago lanceolata-t, Rubus, Galium-t, Caryophyllaceae undiff., Centaurea, Echium, Plantago major/media, Papaveraceae undiff, Dipsacaceae, Vitis, Trifolium-t, Plantago sp., Cirsium-t, Senecio-t, Cichorieae, Brassicaceae SUM, Chenopodiaceae, Rumex, Carduus-t, Apiaceae, Sinapis-t, Lotus-t, Plantago albicans, Plantago coronopus-t, Hornungia-t | |
| Environmental Variable | Df | Variance | F | p-Value |
|---|---|---|---|---|
| Herbivory pressure | 1 | 0.35 | 0.83 | 0.603 |
| Agropastoral use | 1 | 0.72 | 1.69 | 0.057· |
| Trampling | 1 | 0.50 | 1.18 | 0.230 |
| Landscape openness | 1 | 0.73 | 1.72 | 0.036 * |
| EFFIS Fire Occurrence | 1 | 0.51 | 1.21 | 0.250 |
| Distance to urban | 1 | 0.28 | 0.65 | 0.803 |
| Distance to forest | 1 | 1.09 | 2.56 | 0.009 ** |
| Pasture area | 1 | 0.96 | 2.25 | 0.021 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servera-Vives, G.; Mus Amezquita, M.; Snitker, G.; Florenzano, A.; Torri, P.; Ruiz, M.; Mercuri, A.M. Human-Impact Gradients through Anthropogenic Pollen Indicators in a Mediterranean Mosaic Landscape (Balearic Islands). Sustainability 2023, 15, 8807. https://doi.org/10.3390/su15118807
Servera-Vives G, Mus Amezquita M, Snitker G, Florenzano A, Torri P, Ruiz M, Mercuri AM. Human-Impact Gradients through Anthropogenic Pollen Indicators in a Mediterranean Mosaic Landscape (Balearic Islands). Sustainability. 2023; 15(11):8807. https://doi.org/10.3390/su15118807
Chicago/Turabian StyleServera-Vives, Gabriel, Maurici Mus Amezquita, Grant Snitker, Assunta Florenzano, Paola Torri, Maurici Ruiz, and Anna Maria Mercuri. 2023. "Human-Impact Gradients through Anthropogenic Pollen Indicators in a Mediterranean Mosaic Landscape (Balearic Islands)" Sustainability 15, no. 11: 8807. https://doi.org/10.3390/su15118807
APA StyleServera-Vives, G., Mus Amezquita, M., Snitker, G., Florenzano, A., Torri, P., Ruiz, M., & Mercuri, A. M. (2023). Human-Impact Gradients through Anthropogenic Pollen Indicators in a Mediterranean Mosaic Landscape (Balearic Islands). Sustainability, 15(11), 8807. https://doi.org/10.3390/su15118807