Development of Bacterial Augmented Floating Treatment Wetlands System (FTWs) for Eco-Friendly Degradation of Malachite Green Dye in Water
Abstract
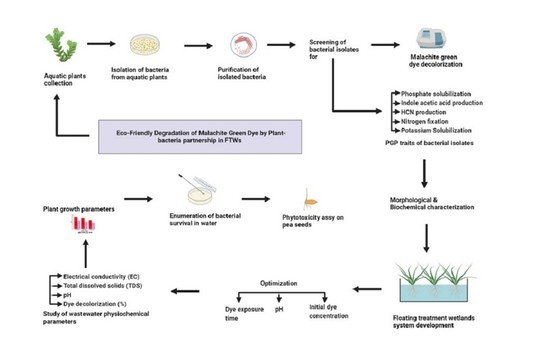
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Collection of Aquatic Plants
2.3. Isolation of Bacteria
2.4. Screening of Bacterial Isolates for MG Dye Decolorization
2.5. Screening of Bacterial Isolates for Plant Growth-Promoting (PGP) Traits
2.6. Morphological and Biochemical Characterization
2.7. Preparation of Floating Treatment Wetlands System (FTWs)
2.7.1. Lab Synthesis of MG Dye Enriched Industrial Wastewater
2.7.2. Macrophytes Used
2.7.3. Bacterial Inoculum Preparation
2.8. Designing and Experimental Setup of FTWs
- Control 1 = Only dye;
- Control 2 = Freshwater and plants;
- T1 = Dye and plants;
- T2 = Dye, plants and bacteria (F1);
- T3 = Dye, plants and bacteria (F2).
2.9. Optimization Studies for Dye Degradation in FTWs
2.10. Study of Physiochemical Parameters of Wastewater in FTWs
2.11. Enumeration of Bacterial Survival in Water
2.12. Plant Growth Monitoring Study
2.13. Chlorophyll and Carotenoids Contents
2.14. Phytotoxicity Assay
3. Results
3.1. Isolation and Screening of MG Dye Decolorizing Bacteria
3.2. Assessment of Bacterial Isolates for Plant Growth-Promoting (PGP) Traits
3.3. Bacterial Isolates Selected for Further Study
3.4. Identification of Selected Bacterial Isolates
3.5. Optimization Studies for MG Dye Decolorization in FTWs
3.6. Physicochemical Parameters of Treated Wastewater
3.7. Enumeration of Bacterial Survival in Water
3.8. Plant Growth Monitoring Study
3.9. Chlorophyll and Carotenoids Content
3.10. Phytotoxicity Assessment of Treated Wastewater
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akaninwor, J.O.; Anosike, E.O.; Egwim, O. Effect of Indomie industrial effluent discharge on microbial properties of new Calabar River. Sci. Res. Essays 2007, 2, 001–005. [Google Scholar]
- Ilyas, M.; Ahmad, W.; Khan, H.; Yousaf, S.; Yasir, M.; Khan, A. Environmental and health impacts of industrial wastewater effluents in Pakistan: A review. Rev. Environ. Health 2019, 34, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Sial, R.; Chaudhary, M.; Abbas, S.; Latif, M.; Khan, A. Quality of effluents from Hattar industrial estate. J. Zhejiang Univ. Sci. B 2006, 7, 974–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaya, V.; Kodi, R.K.; Ninganna, E.; Gowda, B.; Shivanna, M.B. Decolorization of Malachite green dye by Stenotrophomonas maltophilia a compost bacterium. Bull. Natl. Res. Cent. 2021, 45, 81. [Google Scholar] [CrossRef]
- Swan, N.B.; Zaini, M.A.A. Adsorption of malachite green and congo red dyes from water: Recent progress and future outlook. Ecol. Chem. Eng. 2019, 26, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Tewari, K.; Singhal, G.; Arya, R.K. Adsorption removal of malachite green dye from aqueous solution. Rev. Chem. Eng. 2018, 34, 427–453. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Jiang, X.; Yu, J.; Chen, X. Adsorption and removal of malachite green from aqueous solution using magnetic β-cyclodextrin-graphene oxide nanocomposites as adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 166–173. [Google Scholar] [CrossRef]
- Raghukumar, C.; D’Souza-Ticlo, D.; Verma, A.K. Treatment of colored effluents with lignin-degrading enzymes: An emerging role of marine-derived fungi. Crit. Rev. Microbiol. 2008, 34, 189–206. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, S.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Sudova, E.; Machova, J.; Svobodova, Z.; Vesely, T. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: A review. Vet. Med. 2007, 52, 527. [Google Scholar] [CrossRef] [Green Version]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.M.; Afzal, M. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Clean. Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Ajibade, F.O.; Adeniran, K.A.; Egbuna, C.K. Phytoremediation efficiencies of water hyacinth in removing heavy metals in domestic sewage (A Case Study of University of Ilorin, Nigeria). Int. J. Eng. Sci. 2013, 2, 16–27. [Google Scholar]
- Dahake, A.; Hedaoo, M. Application of Water Hyacinth (Eichhornia crassipes) in Wastewater Treatment—A Review. Int. J. Eng. Res. Technol. 2018, 5, 1573–1577. [Google Scholar]
- Mustafa, H.M.; Hayder, G. Recent studies on applications of aquatic weed plants in phytoremediation of wastewater: A review article. Ain Shams Eng. J. 2021, 12, 355–365. [Google Scholar] [CrossRef]
- Smith, M.; Kalin, M. Floating Wetland Vegetation Covers for Suspended Solids Removal. In Quebec 2000 Conference Proceedings: Treatment Wetlands for Water Quality Improvement; CH2M Hill Canada Ltd.: Shawnee Mission, ON, Canada, 2000; pp. 6–12. [Google Scholar]
- Todd, J.; Brown, E.J.; Wells, E. Ecological design applied. Ecol. Eng. 2003, 20, 421–440. [Google Scholar] [CrossRef]
- Headley, T.; Tanner, C.C.; Council, A.R. Application of Floating Wetlands for Enhanced Stormwater Treatment: A Review; Auckland Regional Council Auckland Regional Council Technical Publication: Auckland, New Zealand, 2008; p. 93. Available online: https://www.arc.govt.nz/plans/technical-publications/technical-publications-301-350.cfm (accessed on 13 October 2022).
- Li, H.; Hao, H.L.; Yang, X.E.; Xiang, L.C.; Zhao, F.L.; Jiang, H.H.; He, Z.L. Purification of refinery wastewater by different perennial grasses growing in a floating bed. J. Plant Nutr. 2012, 35, 93–110. [Google Scholar] [CrossRef]
- Arshad, A.; Ali, S.; Khan, S.N.; Riaz, M.; Arshad, S.; Arslan, C.; Noor, S.; Waqas, M.M. Design of floating wetland for treatment of municipal wastewater and environmental assessment using emergy technique. Proc. Int. Acad. Ecol. Environ. Sci. 2017, 7, 78–89. [Google Scholar]
- Ali, A.S.; Lens, P.N.P.; Van Bruggen, J.J.A.H. Purifying municipal wastewater using floating treatment wetlands: Free floating and emergent macrophytes. Adv. Recycl. Waste Manag. 2017, 2, 138. [Google Scholar] [CrossRef]
- Arslan, M.; Imran, A.; Khan, Q.M.; Afzal, M. Plant–bacteria partnerships for the remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2017, 24, 4322–4336. [Google Scholar] [CrossRef]
- Shehzadi, M.; Afzal, M.; Khan, M.U.; Islam, E.; Mobin, A.; Anwar, S.; Khan, Q.M. Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res. 2014, 58, 152–159. [Google Scholar] [CrossRef]
- Saleem, H.; Rehman, K.; Arslan, M.; Afzal, M. Enhanced degradation of phenol in floating treatment wetlands by plant-bacterial synergism. Int. J. Phytoremediat. 2018, 20, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, K.; He, X.; Wang, X. Removal of nitrogen by three plant species in hydroponic culture: Plant uptake and microbial degradation. Water, Air, Soil Pollut. 2016, 227, 324. [Google Scholar] [CrossRef]
- Tondera, K.; Chazarenc, F.; Chagnon, P.L.; Brisson, J. Bioaugmentation of treatment wetlands—A review. Sci. Total Environ. 2021, 775, 145820. [Google Scholar] [CrossRef] [PubMed]
- Raper, E.; Stephenson, T.; Anderson, D.R.; Fisher, R.; Soares, A. Industrial wastewater treatment through bioaugmentation. Process Saf. Environ. Prot. 2018, 118, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Soda, S.; Ike, M.; Fujita, M. Effects of inoculation of a genetically engineered bacterium on performance and indigenous bacteria of a sequencing batch activated sludge process treating phenol. J. Ferment. Bioeng. 1998, 86, 90–96. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Zaida, Z.N.; Piakong, M.T. Bioaugmentation of petroleum hydrocarbon in contaminated soil: A review. In Microbial Action Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 2018; pp. 415–439. [Google Scholar]
- Kabra, A.; Khandare, R.; Govindwar, S. Development of a bioreactor for remediation of textile effluent and dye mixture: A plant-bacterial synergistic strategy. Water Res. 2013, 47, 1035–1048. [Google Scholar] [CrossRef]
- Watharkar, A.D.; Khandare, R.V.; Waghmare, P.R.; Jagadale, A.D.; Govindwar, S.P.; Jadhav, J.P. Treatment of textile effluent in a developed phytoreactor with immobilized bacterial augmentation and subsequent toxicity studies on Etheostoma olmstedi fish. J. Hazard. Mater. 2015, 283, 698–704. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef]
- Gamage, N.; Yapa, P. Use of water Hyacinth (Eichhornia crassipes (Mart) Solms) in treatment systems for textile mill effluents-a case study. J. Natl. Sci. Found. Sri Lanka 2001, 29, 15–28. [Google Scholar] [CrossRef]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediat. 2016, 19, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, A.A.; Naeem, M.; Gill, S.S.; AlZuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Dias, F.S.; do Nascimento, J.P.A.; de Meneses, J.M. Aplicação de macrófitas aquáticas para tratamento de efluente doméstico. Rev. Salud. Ambient. 2016, 2, 106–115. [Google Scholar]
- Qamar, M.T.; Mumtaz, H.M.; Mohsin, M.; Asghar, H.N.; Iqbal, M.; Nasir, M. Development of floating treatment wetlands with plant-bacteria partnership to clean textile bleaching effluent. Ind. Text. 2019, 70, 502–511. [Google Scholar] [CrossRef]
- Tusief, M.Q.; Malik, M.H.; Asghar, H.N.; Mohsin, M. Eco-Friendly Degradation of Blue Reactive Dye Enriched Textile Water by Floating Treatment Wetlands (FTWs) System (Part A). Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2020, 63, 153–161. [Google Scholar] [CrossRef]
- Yoneda, Y.; Yamamoto, K.; Makino, A.; Tanaka, Y.; Meng, X.Y.; Hashimoto, J.; Shin-Ya, K.; Satoh, N.; Fujie, M.; Toyama, T.; et al. Novel plant-associated acidobacteria promotes growth of common floating aquatic plants, duckweeds. Microorganisms 2021, 9, 1133. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, M.; Wei, K.; Ding, J.; Liu, Z.; Zhang, K.-Q.; Huang, X. Decolorizing Activity of Malachite Green and Its Mechanisms Involved in Dye Biodegradation by Achromobacter Xylosoxidans MG1. J. Mol. Microbiol. Biotechnol. 2011, 20, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Akter, K.; Zerin, T.; Banik, A. Biodegradation of Textile Dyes by Bacteria Isolated from Textile Industry Effluents. Stamford J. Microbiol. 2020, 9, 5–8. [Google Scholar] [CrossRef]
- Gupta, R.; Singal, R.; Shankar, A.; Kuhad, R.C.; Saxena, R.K. A modified plate assay for screening phosphate solubilizing microorganisms. J. Gen. Appl. Microbiol. 1994, 40, 255–260. [Google Scholar] [CrossRef]
- Loper, J.; Schroth, M. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 1986, 76, 386–389. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951, 26, 192. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rodriguez, M.M.; Piccoli, P.; Anzuay, M.S.; Baraldi, R.; Neri, L.; Taurian, T.; Ureche, M.A.L.; Segura, D.M.; Cohen, A.C. Native bacteria isolated from roots and rhizosphere of Solanum lycopersicum L. increase tomato seedling growth under a reduced fertilization regime. Sci. Rep. 2020, 10, 15642. [Google Scholar]
- Lorck, H. Production of hydrocyanic acid by bacteria. Physiol. Plant. 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Rajawat, M.V.S.; Singh, S.; Tyagi, S.P.; Saxena, A.K. A modified plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere 2016, 26, 768–773. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Siddique, M.; Ali, S.; Tahseen, R.; Afzal, M. Potentialities of floating wetlands for the treatment of polluted water of river Ravi, Pakistan. Ecol. Eng. 2019, 133, 167–176. [Google Scholar] [CrossRef]
- Hussain, A.; Kamran, M.A.; Javed, M.T.; Hayat, K.; Farooq, M.A.; Ali, N.; Ali, M.; Manghwar, H.; Jan, H.; Chaudhary, H.J. Chaudhary. Individual and combinatorial application of Kocuria rhizophila and citric acid on phytoextraction of multi-metal contaminated soils by Glycine max L. Environ. Exp. Bot. 2019, 159, 23–33. [Google Scholar]
- Ekanayake, M.S.; Manage, P. Green approach for decolorization and detoxification of textile dye—CI Direct Blue 201 using native bacterial strains. Environ. Nat. Resour. J. 2020, 18, 1–8. [Google Scholar]
- Kalyani, D.C.; Patil, P.S.; Jadhav, J.P.; Govindwar, S.P. Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp. SUK1. Bioresour. Technol. 2008, 99, 4635–4641. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tamaki, H.; Matsuzawa, H.; Nigaya, M.; Mori, K.; Kamagata, Y. Microbial community analysis in the roots of aquatic plants and isolation of novel microbes including an organism of the candidate phylum OP10. Microbes Environ. 2012, 27, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, V.; Verma, P. Biodegradation of malachite green by a novel copper tolerant Ochrobactrum pseudogrignonense strain GGUPV1 isolated from copper mine wastewater. Bioresour. Bioprocess. 2015, 2, 42. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.C.; Biswas, S.K.; Sheam, M.M.; Hasan, M.R.; Saha, A.K.; Roy, A.K.; Haque, M.E.; Rahman, M.M.; Tang, S.S. Bioremediation of malachite green dye by two bacterial strains isolated from textile effluents. Curr. Res. Microbiol. Sci. 2020, 1, 37–43. [Google Scholar] [CrossRef]
- Sneha, U.; Poornima, R.; Sridhar, S. Optimization and decolorization of malachite green using Pseudomonas putida. J. Chem. Pharm. Res. 2014, 6, 50–57. [Google Scholar]
- Wanyonyi, W.C.; Onyan, J.M.; Shiundu, P.W.; Mulaa, F.J. Biodegradation and detoxifcation of melachite green dye using novelenzymes from Bacillus cereus strain KM201428: Kinetic and Metabolite Analysis. Energy Procedia 2017, 119, 38–51. [Google Scholar] [CrossRef]
- Fahid, M.; Arslan, M.; Shabir, G.; Younus, S.; Yasmeen, T.; Rizwan, M.; Siddique, K.; Ahmad, S.R.; Tahseen, R.; Iqbal, S.; et al. Phragmites australis in combination with hydrocarbons degrading bacteria is a suitable option for remediation of dieselcontaminated water in floating wetlands. Chemosphere 2020, 240, 124890. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar]
- Wang, L.; Huang, X.; Ma, F.; Ho, S.-H.; Wu, J.; Zhu, S. Role of Rhizophagus irregularis in alleviating cadmium toxicity via improving the growth, micro-and macroelements uptake in Phragmites australis. Environ. Sci. Pollut. Res. 2016, 24, 3593–3607. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, M.S.; Udayanga, D.; Wijesekara, I.; Manage, P. Phytoremediation of synthetic textile dyes: Biosorption and enzymatic degradation involved in efficient dye decolorization by Eichhornia crassipes (Mart.) Solms and Pistia stratiotes L. Environ. Sci. Pollut. Res. 2021, 28, 20476–20486. [Google Scholar] [CrossRef] [PubMed]
- Kagalkar, A.N.; Jagtap, U.B.; Jadhav, J.P.; Govindwar, S.P.; Bapat, V.A. Studies on phytoremediation potentiality of Typhonium flagelliforme for the degradation of Brilliant Blue R. Planta 2010, 232, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Anjana, S.; Thanga, V. Phytoremediation of synthetic textile dyes. Asian J. Microbiol. Biotechnol. Environ. Sci. 2011, 13, 30–39. [Google Scholar]
- Muthunarayanan, V.; Santhiya, M.; Swabna, V.; Geetha, A. Phytodegradation of textile dyes by water hyacinth (Eichhornia crassipes) from aqueous dye solutions. Int. J. Environ. Sci. 2011, 1, 1702–1717. [Google Scholar]
- Kagalkar, A.N.; Jagtap, U.B.; Jadhav, J.P.; Bapat, V.A.; Govindwar, S.P. Biotechnological strategies for phytoremediation of the sulfonated azo dye Direct Red 5B using Blumea malcolmii Hook. Bioresour. Technol. 2009, 100, 4104–4110. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, M.; Zhang, B.-B.; Yu, S.-Y.; Bian, X.-J.; Wang, W.; Wang, Y. Purification and characterization of laccase from Pycnoporus sanguineus and decolorization of an anthraquinone dye by the enzyme. Appl. Microbiol. Biotechnol. 2006, 74, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.R.; Movafeghi, A.; Torbati, S.; Lisar, S.S.; Zarei, M. Phytoremediation potential of duckweed (Lemna minor L.) in degradation of CI Acid Blue 92: Artificial neural network modeling. Ecotoxicol. Environ. Saf. 2012, 80, 291–298. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Z.; Li, X. The use of vetiver grass (Vetiveria zizanioides) in the phytoremediation of soils contaminated with heavy metals. Appl. Geochem. 2004, 19, 1553–1565. [Google Scholar] [CrossRef] [Green Version]
- Ghassemzadeh, F.; Yousefzadeh, H.; Arbab-Zavar, M. Arsenic phytoremediation by Phragmites australis: Green technology. Int. J. Environ. Stud. 2008, 65, 587–594. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Abdel-Ghani, N.T.; El-Chaghaby, G.A. Phytoremediation of industrial wastewater potentiality by Typha domingensis. Int. J. Environ. Sci. Technol. 2011, 8, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Roongtanakiat, N.; Tangruangkiat, S.; Meesat, R. Utilization of vetiver grass (Vetiveria zizanioides) for removal of heavy metals from industrial wastewaters. Sci. Asia 2007, 33, 397–403. [Google Scholar] [CrossRef]
- Joshi, P.; Jaybhaye, S.; Mhatre, K. Biodegradation of dyes using consortium of bacterial strains isolated from textile effluent. Eur. J. Exp. Biol. 2015, 5, 36–40. [Google Scholar]
- Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Abd_Allah, E.F.; et al. Bacterial augmented floating treatment wetlands for efficient treatment of synthetic textile dye wastewater. Sustainability 2020, 12, 3731. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Enhancement of oil field-produced wastewater remediation by bacterially-augmented floating treatment wetlands. Chemosphere 2019, 217, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.C.; Headley, T.R. Components of floating emergent macrophyte treatment wetlands influencing removal of stormwater pollutants. Ecol. Eng. 2011, 37, 474–486. [Google Scholar] [CrossRef]
- Karstens, S.; Nazzari, C.; Bâlon, C.; Bielecka, M.; Grigaitis, Ž.; Schumacher, J.; Stybel, N.; Razinkovas-Baziukas, A. Floating wetlands for nutrient removal in eutrophicated coastal lagoons: Decision support for site selection and permit process. Mar. Policy 2018, 97, 51–60. [Google Scholar] [CrossRef]
- Yousaf, S.; Afzal, M.; Reichenauer, T.G.; Brady, C.L.; Sessitsch, A. Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains. Environ. Pollut. 2011, 159, 2675–2683. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, A.; Shabir, G.; Khan, Q.M.; Afzal, M. Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol. Eng. 2015, 84, 58–66. [Google Scholar] [CrossRef]
- Afzal, M.; Rehman, K.; Shabir, G.; Tahseen, R.; Ijaz, A.; Hashmat, A.J.; Brix, H. Largescale remediation of oil-contaminated water using floating treatment wetlands. NPJ Clean Water 2019, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Afzal, M.; Naveed, M.; Shahid, M.; Ahmad Zahir, Z. Endophytic bacteria enhance remediation of tannery effluent in constructed wetlands vegetated with Leptochloa fusca. Int. J. Phytorem. 2018, 20, 121–128. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef]
- Afridi, M.S.; Amna; Sumaira; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; et al. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Fatima, K.; Imran, A.; Amin, I.; Khan, Q.M.; Afzal, M. Plant species affect colonization patterns and metabolic activity of associated endophytes during phytoremediation of crude oil-contaminated soil. Environ. Sci. Pollut. Res. 2015, 23, 6188–6196. [Google Scholar] [CrossRef]
- Afzal, M.; Shabir, G.; Tahseen, R.; Islam, E.-U.; Iqbal, S.; Khan, Q.M.; Khalid, Z.M. Endophytic burkholderia sp. strain PsJN improves plant growth and phytoremediation of soil irrigated with textile effluent. CLEAN–Soil Air Water 2013, 42, 1304–1310. [Google Scholar] [CrossRef]
- Agarry, S.E.; Oghenejoboh, K.M.; Latinwo, G.K.; Owabor, C.N. Biotreatment of petroleum refinery wastewater in vertical surface-flow constructed wetland vegetated with Eichhornia crassipes: Lab-scale experimental and kinetic modelling. Environ. Technol. 2018, 41, 1793–1813. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Muntaha, S.; Rashid, M.; Sun, G.; Hasnat, A. Industrial wastewater treatment in constructed wetlands packed with construction materials and agricultural by-products. J. Clean. Prod. 2018, 189, 442–453. [Google Scholar] [CrossRef]
- Pajares, E.M.; Valero, L.G.; Sánchez, I.M.R. Cost of Urban Wastewater Treatment and Ecotaxes: Evidence from Municipalities in Southern Europe. Water 2019, 11, 423. [Google Scholar]
- Afzal, M.; Arslan, M.; Müller, J.A.; Shabir, G.; Islam, E.; Tahseen, R.; Anwar-Ul-Haq, M.; Hashmat, A.J.; Iqbal, S.; Khan, Q.M. Floating treatment wetlands as a suitable option for large-scale wastewater treatment. Nat. Sustain. 2019, 2, 863–871. [Google Scholar] [CrossRef]
- Hassan, I.; Chowdhury, S.R.; Prihartato, P.K.; Razzak, S.A. Wastewater Treatment Using Constructed Wetland: Current Trends and Future Potential. Processes 2021, 9, 1917. [Google Scholar] [CrossRef]









| Properties | Malachite Green Dye |
|---|---|
| Chemical structure |  |
| Chemical formula | C23H25ClN2 |
| Molecular weight | 364.9 g/mol |
| Maximum strength | 618 nm |
| Water solubility | 6. 104 mg/dm3 |
| Chemical Safety |  Corrosive Irritant |
| GHS Signal | Danger |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H318 (100%): Causes serious eye damage [Danger Serious eye damage/eye irritation] |
| Other names | Aniline green, Victoria green B, Basic green 4 (BG4), Diamond green B |
| PGP Traits | Bacterial Isolates | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F4 | F7 | F10 | F12 | |
| Phosphate solubilization | − | − | + | − | − | − |
| IAA production | + | + | − | + | ++ | ++ |
| N2 fixation | − | − | − | − | + | − |
| HCN production | ++ | + | ++ | − | + | +++ |
| Potassium solubilization | − | − | − | − | − | − |
| Colony Characters | Bacterial Isolates | |
|---|---|---|
| F1 | F2 | |
| Form | Round | Round |
| Margins | Entire | Entire |
| Elevations | Convex | Convex |
| Size | Moderate | Small |
| Texture | Smooth | Smooth |
| Appearance | Shiny | Shiny |
| Color | Creamy white | Creamy white |
| Optical property | Opaque | Opaque |
| Gram stain | Gram negative | Gram negative |
| Cell shape | Short rods | Short rods |
| Test Name | Bacterial Isolates | |
|---|---|---|
| F1 | F2 | |
| Nitrate reduction (NO3) | − | − |
| Indole production (TRP) | − | − |
| Glucose fermentation (GLU) | − | − |
| Arginine dihydrolase production (ADH) | − | − |
| Urease production (URE) | − | − |
| Β-glucosidase hydrolysis (ESC) | − | − |
| Protease hydrolysis (GEL) | − | − |
| B-galactosidase production (PNPG) | − | − |
| Assimilation of | ||
| Glucose (GLU) | + | + |
| Arabinose (ARA) | − | − |
| Mannose (MNE) | + | + |
| Mannitol (MAN) | − | + |
| N-Acetyl-Glucosamine (NAG) | − | − |
| Maltose (MAL) | − | − |
| Potassium Gluconate (GNT) | + | + |
| Capric acid (CAP) | + | + |
| Adipic acid (ADI) | − | − |
| Malate (MLT) | + | + |
| Trisodium Citrate (CIT) | − | + |
| PhenylAcetic acid (PAC) | + | − |
| Cytochrome oxidase production (OX) | + | + |
| API Codes & organism | Pseudomonas putida (42456) | Pseudomonas sp. (46455) |
| Treatments | Dye Removal | EC | TDS |
|---|---|---|---|
| T1 | 82.71 | 60.09 | 40.1 |
| T2 | 91.58 | 68.75 | 48.89 |
| T3 | 87.39 | 62.9 | 45.84 |
| Floating Wetlands Treatment | Day 1 (CFU)/mL × 103) | Day 4 (CFU)/mL × 103) |
|---|---|---|
| T2 | 219 ± 1.52 | 211 ± 5.00 |
| T3 | 219 ± 1.00 | 210 ± 4.00 |
| Treatments | Total Weight (g) | Stalk Length (cm) | Root Length (cm) |
|---|---|---|---|
| Control 2 | 139.43 ± 0.37 | 32.02 ± 0.01 | 20.08 ± 0.75 |
| T1 | 113.51 ± 0.44 | 25.13 ± 0.11 | 11.26 ± 0.11 |
| T2 | 127.19 ± 0.16 | 26.04 ± 0.04 | 15.06 ± 0.05 |
| T3 | 121.45 ± 0.39 | 22.06 ± 0.05 | 13.64 ± 0.56 |
| Treatments | Germination (%) | Plumule (cm) | Radicle (cm) |
|---|---|---|---|
| Tap water | 99.70 ± 0.52 | 2.40 ± 0.01 | 1.57 ± 0.06 |
| Synthetic wastewater (MG dye) | 30.73 ± 0.58 | 0.77 ± 0.06 | 0.49 ± 0.01 |
| T1 | 92.53 ± 0.15 | 2.37 ± 0.11 | 1.15 ± 0.01 |
| T2 | 96.57 ± 0.21 | 2.36 ± 0.05 | 1.20 ± 0.01 |
| T3 | 94.43 ± 0.06 | 2.37 ± 0.03 | 1.17 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahreen, S.; Mukhtar, H. Development of Bacterial Augmented Floating Treatment Wetlands System (FTWs) for Eco-Friendly Degradation of Malachite Green Dye in Water. Sustainability 2023, 15, 4541. https://doi.org/10.3390/su15054541
Sahreen S, Mukhtar H. Development of Bacterial Augmented Floating Treatment Wetlands System (FTWs) for Eco-Friendly Degradation of Malachite Green Dye in Water. Sustainability. 2023; 15(5):4541. https://doi.org/10.3390/su15054541
Chicago/Turabian StyleSahreen, Sania, and Hamid Mukhtar. 2023. "Development of Bacterial Augmented Floating Treatment Wetlands System (FTWs) for Eco-Friendly Degradation of Malachite Green Dye in Water" Sustainability 15, no. 5: 4541. https://doi.org/10.3390/su15054541
APA StyleSahreen, S., & Mukhtar, H. (2023). Development of Bacterial Augmented Floating Treatment Wetlands System (FTWs) for Eco-Friendly Degradation of Malachite Green Dye in Water. Sustainability, 15(5), 4541. https://doi.org/10.3390/su15054541