Remote Sensing of Coral Bleaching Using Temperature and Light: Progress towards an Operational Algorithm
Abstract
:1. Introduction
Coral Response to Variable Solar Irradiance
2. Methods
2.1. Definition of Relative Fv/Fm
2.2. Photoacclimation
2.3. Definition of EEE
2.4. Definition of HotSpot
2.5. Experimental Quantification of the Synergistic Effect of Light and Temperature
| i.e., at 30 °C rel Fv/Fm = 0.982 − 0.00663 EEE |
| at 32 °C rel Fv/Fm = 0.955 − 0.00837 EEE |
2.6. LSD Algorithm Description
3. Results
3.1. LSD Algorithm Demonstration
3.2. Definitions
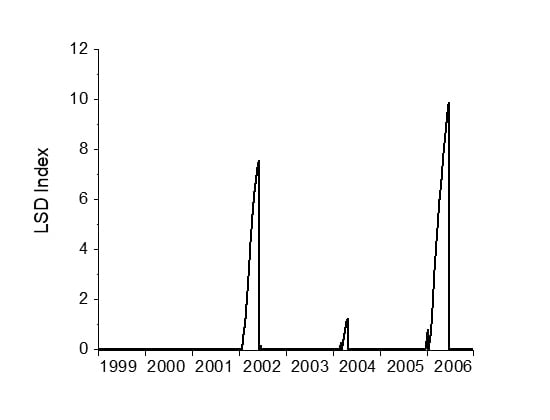
3.3. Keppel Islands Example
3.3.1. Step 1: Derive the Daily Value of rel Fv/Fm Due to EEE with No Temperature Effect
3.3.2. Step 2: Derive the Daily Variation in Fv/Fm Due to EEE (with No Temperature Effect)
3.3.3. Step 3: Derive the Daily Value of rel Fv/Fm Due to EEE, Including Temperature Effects
| i.e., (rel Fv/Fm)i= (1 − 0.01164 HSi) + (−0.00426 − 0.00130 HSi) EEEi |
3.3.4. Step 4: Derive the Daily Variation in the Absolute Values of Fv/Fm Due to EEE, Including Temperature Effects
3.3.5. Step 5: Calculate the Light Stress Damage Index
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glynn, P.W. Coral Reef Bleaching: Facts, Hypothesis and Implications. Glob. Chang. Biol. 1996, 2, 495–509. [Google Scholar]
- Lesser, M.P. Elevated temperature and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 1996, 41, 271–283. [Google Scholar] [CrossRef]
- Brown, B.E. The significance of pollution in eliciting the ‘bleaching’ response in symbiotic cnidarians. Int. J. Environ. Pollut. 2000, 13, 392–415. [Google Scholar] [CrossRef]
- Douglas, A.E. Coral Bleaching—How and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [PubMed]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the World’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar]
- Hoegh-Guldberg, O.; Fine, M.; Skirving, W.; Johnstone, R.; Dove, S.; Strong, A. Coral bleaching following wintry weather. Limnol. Oceanogr. 2005, 50, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Dove, S.G.; Hoegh-Guldberg, O. The cell physiology of coral bleaching. In Coral Reefs and Climate Change: Science and Management; Phinney, J.T., Hoegh-Guldberg, O., Kleypas, J., Skirving, W., Strong, A., Eds.; American Geophysical Union: Washington, DC, USA, 2006; pp. 55–71. [Google Scholar]
- Falkowski, P.G.; Jokiel, P.L.; Kinzie, R.A. Irradiance and Corals. In Coral Reefs; Dubinsky, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 89–107. [Google Scholar]
- Iglesias-Prieto, R.; Trench, R.K. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar. Ecol. Prog. Ser. 1994, 113, 163–175. [Google Scholar]
- Hennige, S.J.; Suggett, D.J.; Warner, M.E.; McDougall, K.E.; Smith, D.J. Photobiology of Symbiodinium revisited: Bio-Physical and bio-optical signatures. Coral Reefs 2009, 28, 179–195. [Google Scholar]
- Dubinsky, Z.; Stambler, N.; Ben-Zion, M.; McCloskey, L.; Muscatine, L.; Falkowski, P. The effect of external nutrient resources on the optical properties and photosynthetic efficiency of Stylphora pistillata. Proc. R. Soc. Lond. 1990, 239, 231–246. [Google Scholar]
- Fagoonee, I.I.; Wilson, H.B.; Hassell, M.P.; Turner, J.R. The dynamics of zooxanthellae populations: A long-term study in the field. Science 1999, 283, 843–845. [Google Scholar]
- Fitt, W.K.; Mcfarland, M.E.; Warner, M.E.; Chilcoat, G.C. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates and relation to coral bleaching. Limnol. Oceanogr. 2000, 45, 677–685. [Google Scholar] [CrossRef]
- Brown, B.E.; Dunne, R.P.; Ambarsari, I.; Le Tissier, M.D.A.; Satapoomin, U. Seasonal fluctuations in environmental factors and variations in symbiotic algae and chlorophyll pigments in four Indo-Pacific coral species. Mar. Ecol. Prog. Ser. 1999, 191, 53–69. [Google Scholar] [CrossRef]
- Berkelmans, R.; De’ath, G.; Kinimonth, S.; Skirving, W. Coral bleaching on the Great Barrier Reef: Correlation with sea surface temperature, a handle on ‘patchiness’ and comparison of the 1998 and 2002 events. Coral Reefs 2004, 23, 74–83. [Google Scholar] [CrossRef]
- Glynn, P.W. Widespread coral mortality and the 1982–83 El Niño warming event. Environ. Conserv. 1984, 11, 133–146. [Google Scholar] [CrossRef]
- Coffroth, M.A.; Lasker, H.R.; Oliver, J.E. Coral mortality outside of the eastern Pacific during 1982–1983: Relationship to El Niño. In Global Ecological Consequences of the 1982–83 El Niño-Southern Oscillation; Glynn, P.W., Ed.; Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1989; pp. 141–182. [Google Scholar]
- Wilkinson, C. (Ed.) Status of Coral Reefs of the World; Australian Institute of Marine Science: Townsville, Queensland, Australia, 2004. [Google Scholar]
- Eakin, M.C.; Liu, G.; Gomez, A.M.; De La Cour, J.L.; Heron, S.F.; Skirving, W.J.; Geiger, E.F.; Marsh, B.L.; Tirak, K.V.; Strong, A.E. Ding, Dong, The Witch is Dead (?)—Three Years of Global Coral Bleaching 2014–2017. Reef Encount. 2017, 32, 33–38. [Google Scholar]
- Skirving, W.J.; Strong, A.E.; Liu, G.; Liu, C.; Arzayus, F.; Sapper, J.; Bayler, E. Extreme events and perturbations of coastal ecosystems: Sea surface temperature change and coral bleaching. In Remote Sensing of Aquatic Coastal Ecosystem Processes; Richardson, L.L., LeDrew, E.F., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 11–25. [Google Scholar]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, 129–138. [Google Scholar] [CrossRef]
- Liu, G.; Heron, S.F.; Eakin, C.M.; Muller-Karger, F.E.; Vega-Rodriguez, M.; Guild, L.S.; De La Cour, J.L.; Geiger, E.F.; Skirving, W.J.; Burgess, T.F.R.; et al. Reef-scale Thermal Stress Monitoring of Coral Ecosystems: New 5-km Global Products from NOAA Coral Reef Watch. Remote Sens. 2014, 6, 11579–11606. [Google Scholar] [CrossRef]
- Liu, G.; Skirving, W.J.; Geiger, E.F.; De La Cour, J.L.; Marsh, B.L.; Heron, S.F.; Tirak, K.V.; Strong, A.E.; Eakin, C.M. NOAA Coral Reef Watch’s 5 km Satellite Coral Bleaching Heat Stress Monitoring Product Suite Version 3 and Four-Month Outlook Version 4. Reef Encount. 2017, 32, 39–45. [Google Scholar]
- Eakin, C.M.; Morgan, J.A.; Heron, S.F.; Smith, T.B.; Liu, G.; Alvarez-Filip, L.; Baca, B.; Bartels, E.; Bastidas, C.; Bouchon, C.; et al. Caribbean Corals in Crisis: Record Thermal Stress, Bleaching and Mortality in 2005. PLoS ONE 2010, 5, e13969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heron, S.F.; Johnston, L.; Liu, G.; Geiger, E.F.; Maynard, J.A.; De La Cour, J.L.; Johnson, S.; Okano, R.; Benavente, D.; Burgess, T.F.R.; et al. Validation of Reef-scale Thermal Stress Satellite Products for Coral Bleaching Monitoring. Remote Sens. 2016, 8, 59. [Google Scholar] [CrossRef]
- Kayanne, H. Validation of degree heating weeks as a coral bleaching index in the northwestern Pacific. Coral Reefs 2017, 36, 63. [Google Scholar] [CrossRef]
- Iglesias-Prieto, R.; Matta, J.L.; Robins, W.A.; Trench, R.K. Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc. Natl. Acad. Sci. USA 1992, 89, 10302–10305. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Hoegh-Guldberg, O.; Larkum, A.W.D.; Schreiber, U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 1998, 21, 1219–1230. [Google Scholar] [CrossRef]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl. Acad. Sci. USA 1999, 96, 8007–8012. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Nakamura, T.; Sakamizu, M.; van Woesik, R.; Yamasaki, H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Phys. 2004, 45, 251–255. [Google Scholar] [CrossRef]
- Mumby, P.J.; Chisholm, J.R.M.; Edwards, A.J.; Andrefouet, S.; Jaubert, J. Cloudy weather may have saved Society Island reef corals during the 1998 ENSO event. Mar. Ecol. Prog. Ser. 2001, 222, 209–216. [Google Scholar] [CrossRef]
- Niyogi, K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 6, 455–460. [Google Scholar] [CrossRef]
- Anderson, J.M.; Chow, W.S.; Park, Y.I. The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynth. Res. 1995, 46, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chalker, B. Simulating light-saturation curves for photosynthesis and calcification by reef-building corals. Mar. Biol. 1981, 63, 135–141. [Google Scholar] [CrossRef]
- Melis, A. Photosystem II damage and repair cycle in chloroplasts: What modulates the rate of photodamage “in vivo”? Trends Plant Sci. 1999, 94, 130–135. [Google Scholar] [CrossRef]
- Vass, I.; Styring, S.; Hundal, T.; Koivuniemi, A.; Aro, E.; Andersson, B. Reversible and irreversible intermediates during photoinhibition of photosystem II: Stable reduced QA species promote chlorophyll triplet formation. Proc. Natl. Acad. Sci. USA 1992, 90, 1408–1412. [Google Scholar] [CrossRef]
- Vásquez-Elizondo, R.M.; Enríquez, S. Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Sci. Rep. 2016, 6, 19030. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Brown, C.M.; DeZeeuw, K.; Campbell, D.A.; Ralph, P.J. Increased rate of D1 repair in coral symbionts during bleaching is insufficient to counter accelerated photo-inactivation. Limnol. Oceanogr. 2011, 56, 139–146. [Google Scholar] [CrossRef]
- Weis, V.M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 2008, 211, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Titlyanov, E.A.; Titlyanova, T.V.; Leletkin, V.A.; Tsukahara, J.; van Woesik, R.; Yamazato, K. Degradation of zooxanthellae and regulation of their density in hermatypic corals. MEPS 2006, 139, 167–178. [Google Scholar] [CrossRef]
- Dunn, S.R.; Pernice, M.; Green, K.; Hoegh-Guldberg, O.; Dove, S.G. Thermal Stress Promotes Host Mitochondrial Degradation in Symbiotic Cnidarians: Are the Batteries of the Reef Going to Run Out? PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.A.; Eakin, C.M.; Friedman, C.S.; Froelich, B.; Hershberger, P.K.; Hofmann, E.E.; Petes, L.E.; Prager, K.C.; Weil, E.; Willis, B.L.; et al. Climate Change Influences on Marine Infectious Diseases: Implications for Management and Society. Ann. Rev. Mar. Sci. 2014, 6, 249–277. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Prieto, R. Temperature-dependent inactivation of photosystem II in symbiotic dinoflagellates. In Proceedings of the 8th International Coral Reef Symposium, Panama City, Panama, 24–29 June 1996; Macintyre, I.G., Ed.; Smithsonian Tropical Research Institute: Balboa, Panama, 1997; pp. 1313–1318. [Google Scholar]
- Warner, M.E.; LaJeunesse, T.C.; Robinson, J.D.; Thur, R.M. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: Potential implications for coral bleaching. Limnol. Oceanogr. 2006, 51, 1887–1897. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin, Germany, 1995; pp. 49–70. [Google Scholar]
- Rodríguez-Román, A.; Hernández-Pech, X.; Thomé, P.E.; Enríquez, S.; Iglesias-Prieto, R. Photosynthesis and light utilization in the Caribbean coral Montastraea faveolata recovering from a bleaching event. Limnol. Oceanogr. 2016, 51, 2702–2710. [Google Scholar] [CrossRef]
- DeSalvo, M.K.; Sunagawa, S.; Fisher, P.; Voolstra, C.R.; Iglesias-Prieto, R.; Medina, M. Coral host transcriptomic states are correlated with Symbiodinium genotypes. Mol. Ecol. 2010, 19, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.P.; Falk, S.; Huner, N.P.A. Photosystem II excitation pressure and development of resistance to photoinhibition. I. Light-harvesting complex II abundance and zeaxanthin content in Chlorella vulgaris. Plant Phys. 1995, 107, 687–694. [Google Scholar] [CrossRef]
- Iglesias-Prieto, R.; Beltrán, V.H.; LaJeunesse, T.C.; Reyes-Bonilla, H.; Thomé, P.E. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. Lond. 2004, 271, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant Cell Environ. 1996, 19, 291–299. [Google Scholar] [CrossRef]
- Matsubara, S.; Chow, W.S. Populations of photoinactivated photosystem II reaction centers characterized by chlorophyll a fluorescence lifetime in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 18234–18239. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, K.; Nagai, A.; Ueno, S. Examination of the effect of temperature, light intensity and zooxanthellae concentration on calcification and photosynthesis of scleractinian coral Acropora pulchra. J. Sch. Mar. Sci. Technol. Tokai Univ. 1995, 40, 95–103. [Google Scholar]
- Rodolfo-Metalpa, R.; Huot, Y.; Ferrier-Pagès, C. Photosynthetic response of the Mediterranean zooxanthellate coral Cladocora caespitosa to the natural range of light and temperature. J. Exp. Biol. 2008, 211, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, P.J.; Brown, D.; Moriarty, V. Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Glob. Chang. Biol. 2012, 18, 2173–2183. [Google Scholar] [CrossRef]
- Warner, M.E.; Chilcoat, G.C.; McFarland, F.K.; Fitt, W.K. Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Mar. Biol. 2002, 141, 31–38. [Google Scholar]
- Anthony, K.R.N.; Hoegh-Guldberg, O. Kinetics of photoacclimation in corals. Oecologia 2003, 134, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Scheufen, T.; Kramer, W.E.; Iglesias-Prieto, R.; Enríquez, S. Seasonal variation modulates coral sensibility to heat-stress and explains annual changes in coral productivity. Sci. Rep. 2017, 7, 4937. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.F.; Liu, G.; Eakin, C.M.; Skirving, W.J.; Muller-Karger, F.E.; Vega-Rodriguez, M.; De La Cour, J.L.; Burgess, T.F.R.; Strong, A.E.; Geiger, E.F.; et al. Climatology Development for NOAA Coral Reef Watch’s 5-km Product Suite; NOAA Technical Report NESDIS 145; NOAA/NESDIS: College Park, MD, USA, 2015.
- Liu, G.; Rauenzahn, J.L.; Heron, S.F.; Eakin, C.M.; Skirving, W.J.; Christensen, T.R.L.; Strong, A.E.; Li, J. NOAA Coral Reef Watch 50 km Satellite Sea Surface Temperature-Based Decision Support System for Coral Bleaching Management; NOAA Technical Report NESDIS 143; NOAA/NESDIS: College Park, MD, USA, 2013.
- Cooper, T.F.; Uthicke, S.; Humphrey, C.; Fabricius, K.E. Gradients in water column nutrients, sediment parameters, irradiance and coral reef development in the Whitsunday Region, central Great Barrier Reef. Estuar. Coast. Shelf Sci. 2007, 27, 503–519. [Google Scholar] [CrossRef]
- Ignatov, A.; Zhou, X.; Petrenko, B.; Liang, X.; Kihai, Y.; Dash, P.; Stroup, J.; Sapper, J.; DiGiacomo, P. AVHRR GAC SST Reanalysis Version 1 (RAN1). Remote Sens 2016, 8, 315. [Google Scholar] [CrossRef]
- Merchant, C.J.; Embury, O.; Roberts-Jones, J.; Fiedler, E.; Bulgin, C.E.; Corlett, G.K.; Good, S.; McLaren, A.; Rayner, N.; Morak-Bozzo, S.; et al. Sea surface temperature datasets for climate applications from Phase 1 of the European Space Agency Climate Change Initiative (SST CCI). Geosci. Data J. 2014, 1, 179–191. [Google Scholar] [CrossRef]







| Symbol | Description | Units |
|---|---|---|
| i | Day number | day |
| PARi | Daily integrated Photosynthetically Available Radiation on day i | mol quanta m−2 day−1 |
| acclim PARi | PAR to which the corals are currently acclimated on day i | mol quanta m−2 day−1 |
| EEEi | Daily Excess Excitation Energy on day i | mol quanta m−2 day−1 |
| Fv/Fm | diurnal maximum PSII photochemical efficiency | - |
| rel Fv/Fm | change in Fv/Fm relative to yesterday’s Fv/Fm = (Fv/Fm)i/(Fv/Fm)i−1 | - |
| SST | Sea Surface Temperature | °C |
| MMM | Maximum Monthly Mean SST | °C |
| HS | HotSpot = SST − MMM | °C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skirving, W.; Enríquez, S.; Hedley, J.D.; Dove, S.; Eakin, C.M.; Mason, R.A.B.; De La Cour, J.L.; Liu, G.; Hoegh-Guldberg, O.; Strong, A.E.; et al. Remote Sensing of Coral Bleaching Using Temperature and Light: Progress towards an Operational Algorithm. Remote Sens. 2018, 10, 18. https://doi.org/10.3390/rs10010018
Skirving W, Enríquez S, Hedley JD, Dove S, Eakin CM, Mason RAB, De La Cour JL, Liu G, Hoegh-Guldberg O, Strong AE, et al. Remote Sensing of Coral Bleaching Using Temperature and Light: Progress towards an Operational Algorithm. Remote Sensing. 2018; 10(1):18. https://doi.org/10.3390/rs10010018
Chicago/Turabian StyleSkirving, William, Susana Enríquez, John D. Hedley, Sophie Dove, C. Mark Eakin, Robert A. B. Mason, Jacqueline L. De La Cour, Gang Liu, Ove Hoegh-Guldberg, Alan E. Strong, and et al. 2018. "Remote Sensing of Coral Bleaching Using Temperature and Light: Progress towards an Operational Algorithm" Remote Sensing 10, no. 1: 18. https://doi.org/10.3390/rs10010018
APA StyleSkirving, W., Enríquez, S., Hedley, J. D., Dove, S., Eakin, C. M., Mason, R. A. B., De La Cour, J. L., Liu, G., Hoegh-Guldberg, O., Strong, A. E., Mumby, P. J., & Iglesias-Prieto, R. (2018). Remote Sensing of Coral Bleaching Using Temperature and Light: Progress towards an Operational Algorithm. Remote Sensing, 10(1), 18. https://doi.org/10.3390/rs10010018