Time-Series Multispectral Indices from Unmanned Aerial Vehicle Imagery Reveal Senescence Rate in Bread Wheat
Abstract
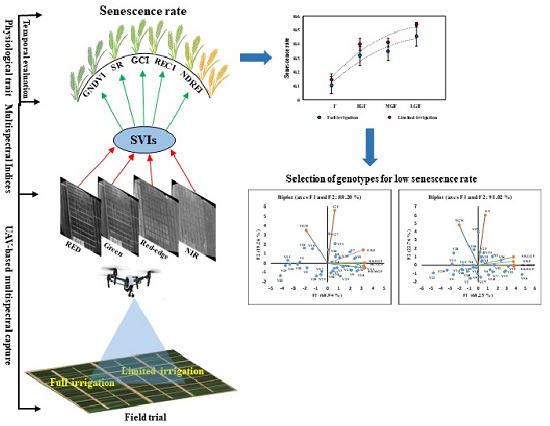
:1. Introduction
2. Material and Methods
2.1. Germplasm
2.2. Experimental Design
2.3. UAV Platform and Flight Mission
2.4. Data Acquisition Schedule
2.5. Image Processing and Data Extraction
2.6. Estimation of Narrowband SVIs and Senescence Rate
2.7. Collection of Ground Morphological Data
2.8. Statistical Analysis
3. Results
3.1. Accuracy of SVI Data to Predict Growth Status
3.2. Estimates of Variance Components and Correlations among SVIs and Yield Traits
3.3. Dynamics and Interaction of SVIs during Different Growth Stages
3.4. Impact of Senescence Rate on Yield and Performance of Genotypes
4. Discussion
4.1. Validation of SVI Data at Different Growth Stages
4.2. Genotypic Variation and Traits Correlation
4.3. The Dynamics of SVIs Explained Interaction between Growth Stages
4.4. Impact of Senescence Rate on Yield
4.5. Cultivars with Low Senescence Rate
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Chl | Chlorophyll |
| CT | Canopy temperature |
| EGF | Early grain filling |
| F | Flowering |
| GCI | Green chlorophyll index |
| GNDVI | Green normalized difference vegetation index |
| KPS | Kernels per spike |
| GS | Growth stages |
| GY | Grain yield |
| H | Heading |
| LAI | Leaf area index |
| LGF | Late grain filling |
| MGF | Mid grain filling |
| NDREI | Normalized difference red-edge index |
| RECI | Red-edge chlorophyll index |
| SR | Simple ratio |
| TGW | Thousand grain weight |
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.; Tattaris, M.; Cossani, C.M.; Ellis, M.; Yamaguchi-Shinozaki, K.; Pierre, C.S. Exploring genetic resources to increase adaptation of wheat to climate change. In Advances in Wheat Genetics: From Genome to Field: 12th International Wheat Genetics Symposium; Ogihara, Y., Takumi, S., Handa, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 355–368. [Google Scholar]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Singh, R.P.; Crossa, J.; Huerta-Espino, J.; Sharma, I.; Chatrath, R.; Singh, G.P.; Sohu, V.S.; Mavi, G.S.; Sukuru, V.S.P.; et al. Earliness in wheat: A key to adaptation under terminal and continual high temperature stress in south Asia. Field Crops Res. 2013, 151, 19–26. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Ding, H.; Qin, H.; Hou, J.; Huang, X.; Xie, Y.; Guo, T. Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front. Plant Sci. 2017, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, K.; Fritz, A.K.; Paulsen, G.M.; Bai, G.; Pandravada, S.; Gill, B.S. Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol. Breed. 2010, 26, 163–175. [Google Scholar] [CrossRef]
- Chapman, S.C.; Cooper, M.; Hammer, G.L. Using crop simulation to generate genotype by environment interaction effects for sorghum in water-limited environments. Aust. J. Agric. Res. 2002, 53, 379–389. [Google Scholar] [CrossRef]
- Kyratzis, A.C.; Skarlatos, D.P.; Menexes, G.C.; Vamvakousis, V.F.; Katsiotis, A. Assessment of vegetation indices derived by UAV imagery for durum wheat phenotyping under a water limited and heat stressed mediterranean environment. Front. Plant Sci. 2017, 8, 1114. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.K.; van Oosterom, E.J.; Mullet, J.E.; George-Jaeggli, B.; Jordan, D.R.; Klein, P.E.; Hammer, G.L. Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytol. 2014, 203, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Sai Prasad, S.V.; Jajoo, A.; Ambati, D. Canopy temperature as a selection parameter for grain yield and its components in durum wheat under terminal heat stress in late sown conditions. Agric. Res. 2015, 4, 238–244. [Google Scholar] [CrossRef]
- Pinto, R.S.; Lopes, M.S.; Collins, N.C.; Reynolds, M.P. Modelling and genetic dissection of stay-green under heat stress. Theor. Appl. Genet. 2016, 129, 2055–2074. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.K.; Mullet, J.E.; George-Jaeggli, B.; van Oosterom, E.J.; Hammer, G.L.; Klein, P.E.; Jordan, D.R. Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J. Exp. Bot. 2014, 65, 6251–6263. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Reynolds, M.P. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 2012, 63, 3789–3798. [Google Scholar] [CrossRef] [PubMed]
- Deery, D.; Jimenez-Berni, J.; Jones, H.; Sirault, X.; Furbank, R. Proximal remote sensing buggies and potential applications for field-based phenotyping. Agronomy 2014, 4, 349–374. [Google Scholar] [CrossRef]
- Jin, X.; Li, Z.; Feng, H.; Xu, X.; Yang, G. Newly combined spectral indices to improve estimation of total leaf chlorophyll content in cotton. IEEE J. Sel. Top. Appl. Res. 2014, 7, 4589–4600. [Google Scholar] [CrossRef]
- Jin, X.; Yang, G.; Xu, X.; Yang, H.; Feng, H.; Li, Z.; Shen, J.; Lan, Y.; Zhao, C. Combined multi-temporal optical and radar parameters for estimating LAI and biomass in winter wheat using HJ and radarsar-2 data. Remote Sens. 2015, 7, 13251–13272. [Google Scholar] [CrossRef]
- Magney, T.S.; Eitel, J.U.; Huggins, D.R.; Vierling, L.A. Proximal NDVI derived phenology improves in-season predictions of wheat quantity and quality. Agric. For. Meteorol. 2016, 217, 46–60. [Google Scholar] [CrossRef]
- Tattaris, M.; Reynolds, M.P.; Chapman, S.C. A direct comparison of remote sensing approaches for high-throughput phenotyping in plant breeding. Front. Plant Sci. 2016, 7, 1131. [Google Scholar] [CrossRef] [PubMed]
- Rutkoski, J.; Poland, J.; Mondal, S.; Autrique, E.; Pérez, L.G.; Crossa, J.; Reynolds, M.; Singh, R. Canopy temperature and vegetation indices from high-throughput phenotyping improve accuracy of pedigree and genomic selection for grain yield in wheat. G3 Genes Genomes Genet. 2016, 6, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating biomass of barley using crop surface models (csms) derived from UAV-based RGB imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Verger, A.; Vigneau, N.; Chéron, C.; Gilliot, J.-M.; Comar, A.; Baret, F. Green area index from an unmanned aerial system over wheat and rapeseed crops. Remote Sens. Environ. 2014, 152, 654–664. [Google Scholar] [CrossRef]
- Jin, X.; Liu, S.; Baret, F.; Hemerlé, M.; Comar, A. Estimates of plant density of wheat crops at emergence from very low altitude UAV imagery. Remote Sens. Environ. 2017, 198, 105–114. [Google Scholar] [CrossRef]
- Guillen-Climent, M.L.; Zarco-Tejada, P.J.; Berni, J.A.J.; North, P.R.J.; Villalobos, F.J. Mapping radiation interception in row-structured orchards using 3d simulation and high-resolution airborne imagery acquired from a UAV. Precis. Agric. 2012, 13, 473–500. [Google Scholar] [CrossRef]
- Sankaran, S.; Khot, L.R.; Carter, A.H. Field-based crop phenotyping: Multispectral aerial imaging for evaluation of winter wheat emergence and spring stand. Comput. Electron. Agric. 2015, 118, 372–379. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shang, J.; Vachon, P.W.; Mcnairn, H. Multiyear Crop Monitoring Using Polarimetric RADARSAT-2 Data. IEEE Trans. Geosci. Remote Sens. 2013, 51, 2227–2240. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, E.; Li, Z.; Zhao, L.; Ji, Y.; Zhang, Y.; Liu, Z. Rape (Brassica napus L.) growth monitoring and mapping based on radarsat-2 time-series data. Remote Sens. 2018, 10, 206. [Google Scholar] [CrossRef]
- Potgieter, A.B.; George-Jaeggli, B.; Chapman, S.C.; Laws, K.; Suárez Cadavid, L.A.; Wixted, J.; Watson, J.; Eldridge, M.; Jordan, D.R.; Hammer, G.L. Multi-spectral imaging from an unmanned aerial vehicle enables the assessment of seasonal leaf area dynamics of sorghum breeding lines. Front. Plant Sci. 2017, 8, 1532. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Candón, D.; De Castro, A.I.; López-Granados, F. Assessing the accuracy of mosaics from unmanned aerial vehicle (uav) imagery for precision agriculture purposes in wheat. Precis. Agric. 2014, 15, 44–56. [Google Scholar] [CrossRef]
- Haghighattalab, A.; Pérez, L.G.; Mondal, S.; Singh, D.; Schinstock, D.; Rutkoski, J.; Ortiz-Monasterio, I.; Singh, R.P.; Goodin, D.; Poland, J. Application of unmanned aerial systems for high throughput phenotyping of large wheat breeding nurseries. Plant Methods 2016, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Gao, F.; Ma, D.; Yin, G.; Rasheed, A.; Dong, Y.; Xiao, Y.; Xia, X.; Wu, X.; He, Z. Genetic progress in grain yield and physiological traits in chinese wheat cultivars of Southern Yellow and Huai Valley since 1950. Crop Sci. 2017, 57, 760–773. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Sehgal, D.; Skot, L.; Singh, R.; Srivastava, R.K.; Das, S.P.; Taunk, J.; Sharma, P.C.; Pal, R.; Raj, B.; Hash, C.T.; et al. Exploring potential of pearl millet germplasm association panel for association mapping of drought tolerance traits. PLoS ONE 2015, 10, e0122165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gizaw, S.A.; Garland-Campbell, K.; Carter, A.H. Use of spectral reflectance for indirect selection of yield potential and stability in Pacific Northwest winter wheat. Field Crops Res. 2016, 196, 199–206. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.; Guo, Y.; Zheng, B. Dynamic monitoring of NDVI in wheat agronomy and breeding trials using an unmanned aerial vehicle. Field Crops Res. 2017, 210, 71–80. [Google Scholar] [CrossRef]
- Gutierrez, M.; Reynolds, M.P.; Raun, W.R.; Stone, M.L.; Klatt, A.R. Spectral water indices for assessing yield in elite bread wheat genotypes under well-irrigated, water-stressed, and high-temperature conditions. Crop Sci. 2010, 50, 197–214. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bu, H.; Denton, A.; Franzen, D.W. Active-optical sensors using red NDVI compared to red edge NDVI for prediction of corn grain yield in North Dakota, U.S.A. Sensors 2015, 15, 27832–27853. [Google Scholar] [CrossRef] [PubMed]
- Christopher, J.T.; Christopher, M.J.; Borrell, A.K.; Fletcher, S.; Chenu, K. Stay-green traits to improve wheat adaptation in well-watered and water-limited environments. J. Exp. Bot. 2016, 67, 5159–5172. [Google Scholar] [CrossRef] [PubMed]
- Montazeaud, G.; Karatoğma, H.; Özturk, I.; Roumet, P.; Ecarnot, M.; Crossa, J.; Özer, E.; Özdemir, F.; Lopes, M.S. Predicting wheat maturity and stay–green parameters by modeling spectral reflectance measurements and their contribution to grain yield under rainfed conditions. Field Crops Res. 2016, 196, 191–198. [Google Scholar] [CrossRef]
- Montesinos-López, O.A.; Montesinos-López, A.; Crossa, J.; de los Campos, G.; Alvarado, G.; Mondal, S.; Rutkoski, J.; González-Pérez, L.; Burgueño, J. Predicting grain yield using canopy hyperspectral reflectance in wheat breeding data. Plant Methods 2017, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Gaju, O.; Reynolds, M.P.; Sparkes, D.L.; Mayes, S.; Ribas-Vargas, G.; Crossa, J.; Foulkes, M.J. Relationships between physiological traits, grain number and yield potential in a wheat DH population of large spike phenotype. Field Crops Res. 2014, 164, 126–135. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: Advantages and limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Tuberosa, R. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, Y.-L.; Wang, T.; Turner, N.C.; Yang, R.-P.; Jin, Y.; Xi, Y.; Zhang, C.; Cui, T.; Fang, X.-W.; et al. Conserved water use improves the yield performance of soybean (Glycine max (L.) Merr.) under drought. Agric. Water Manag. 2017, 179, 236–245. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X.; et al. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: Current status and perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Haghighattalab, A.; Crain, J.; Mondal, S.; Rutkoski, J.; Singh, R.P.; Poland, J. Application of geographically weighted regression to improve grain yield prediction from unmanned aerial system imagery. Crop Sci. 2017, 57, 2478–2489. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wang, H.; Dong, H.; Qi, X.; Zhao, M.; Fang, Y.; Gao, C.; Hu, L. Progress in genetic improvement of grain yield and related physiological traits of Chinese wheat in Henan province. Field Crops Res. 2016, 199, 117–128. [Google Scholar] [CrossRef]
- Gourdji, S.M.; Mathews, K.L.; Reynolds, M.; Crossa, J.; Lobell, D.B. An assessment of wheat yield sensitivity and breeding gains in hot environments. Process. R. Soc. B Biol. Sci. 2013, 280, 20122190. [Google Scholar] [CrossRef] [PubMed]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef] [PubMed]








| Time Point | Growth Stage | Zadok’s Stage | Number of Flights | Altitude | Ground Data |
|---|---|---|---|---|---|
| 1 | Heading | GS-57 | 3 | 40 m | |
| 2 | Flowering | GS-65 | 3 | 30 m | Chl. |
| 3 | Early grain filling | GS-73 | 3 | 30 m | Chl., CT |
| 4 | Mid grain filling | GS-85 | 2 | 30 m | Chl., CT, LAI |
| 5 | Late grain filling | GS-91 | 3 | 30 m | Chl., CT, LAI |
| 6 | Maturity | no | KPS, TGW, GY |
| SVI | Band | Application | Reference |
|---|---|---|---|
| GNDVI | (RNIR − Rgreen)/ (RNIR + Rgreen) | Greenness, LAI | [32] |
| SR | RNIR/Rred | Biomass, LAI | [32] |
| GCI | (RNIR/Rgreen) − 1 | Chlorophyll | [33] |
| RECI | (RNIR/Rred edge) − 1 | Chlorophyll | [33] |
| NDREI | (Rred-edge − Rgreen)/ (Rred-edge − Rgreen) | Greenness |
| Trait | Genotype | Treatment | G × E | H2 | H2 |
|---|---|---|---|---|---|
| F. Value | F. Value | F. Value | Full Irrigation | Limited Irrigation | |
| GNDVI.H | 19.61 *** | 240.08 *** | 3.01 ** | 0.97 | 0.94 |
| GNDVI.F | 8.42 *** | 908.61 *** | 1.94 * | 0.96 | 0.85 |
| GNDVI.EGF | 3.20 *** | 715.80 *** | 1.14 | 0.83 | 0.74 |
| GNDVI.MGF | 2.09 * | 962.91 *** | 1.19 | 0.75 | 0.75 |
| GNDVI.LGF | 2.00 * | 920.09 *** | 1.87 * | 0.80 | 0.71 |
| SR.H | 11.285 *** | 114.22 *** | 1.96 * | 0.93 | 0.93 |
| SR.F | 5.90 *** | 1193.27 *** | 1.55 | 0.93 | 0.83 |
| SR.EGF | 2.72 ** | 973.75 *** | 1.41 | 0.74 | 0.69 |
| SR.MGF | 1.71 * | 847.64 *** | 1.38 | 0.69 | 0.65 |
| SR.LGF | 2.09 * | 1169.01 *** | 1.83 * | 0.69 | 0.69 |
| GCI.H | 19.57 *** | 267.09 *** | 3.58 *** | 0.97 | 0.95 |
| GCI.F | 9.72 *** | 1024.75 *** | 2.61 ** | 0.96 | 0.85 |
| GCI.EGF | 3.08 *** | 700.30 *** | 1.50 | 0.82 | 0.76 |
| GCI.MGF | 2.37 ** | 851.83 *** | 1.56 | 0.77 | 0.74 |
| GCI.LGF | 2.44 ** | 819.46 *** | 2.38 ** | 0.81 | 0.70 |
| RECI.H | 13.46 *** | 465.85 *** | 3.05 *** | 0.97 | 0.90 |
| RECI.F | 13.65 *** | 735.97 *** | 3.16 *** | 0.97 | 0.88 |
| RECI.EGF | 3.81 *** | 738.74 *** | 1.81 * | 0.85 | 0.78 |
| RECI.MGF | 2.52 ** | 662.47 *** | 1.47 | 0.80 | 0.79 |
| RECI.LGF | 3.13 *** | 995.91 *** | 2.39 ** | 0.83 | 0.84 |
| NDREI.H | 3.13 | 367.76 *** | 2.85 ** | 0.91 | 0.90 |
| NDREI.F | 28.94 *** | 582.26 *** | 1.20 | 0.90 | 0.83 |
| NDREI.EGF | 6.71 *** | 563.87 *** | 0.92 | 0.74 | 0.69 |
| NDREI.MGF | 4.24 *** | 1031.39 *** | 1.19 | 0.69 | 0.65 |
| NDREI.LGF | 2.123 ** | 640.66 *** | 1.72 * | 0.69 | 0.69 |
| Chl.F | 1.762 * | 181.83 *** | 1.26 | 0.81 | 0.82 |
| Chl.EGF | 6.33 *** | 1576.50 *** | 1.45 | 0.88 | 0.91 |
| Chl.LGF | 4.45 *** | 590.04 *** | 1.34 | 0.86 | 0.84 |
| CT.EGF | 3.38 *** | 1243.28 *** | 1.37 | 0.77 | 0.89 |
| CT.MGF | 1.31 | 598.65 *** | 0.67 | 0.64 | 0.65 |
| CT.LGF | 3.32 *** | 549.17 *** | 2.17 ** | 0.69 | 0.86 |
| LAI.MGF | 3.83 *** | 405.43 *** | 1.74 * | 0.84 | 0.87 |
| LAI.LGF | 6.88 *** | 527.30 *** | 3.61 *** | 0.90 | 0.84 |
| KPS | 7.41 *** | 14.28 ** | 1.66 * | 0.92 | 0.87 |
| TGW | 12.28 *** | 296.60 *** | 1.60 | 0.88 | 0.95 |
| GY | 2.72 ** | 574.02 *** | 0.98 | 0.75 | 0.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.A.; Yang, M.; Rasheed, A.; Jin, X.; Xia, X.; Xiao, Y.; He, Z. Time-Series Multispectral Indices from Unmanned Aerial Vehicle Imagery Reveal Senescence Rate in Bread Wheat. Remote Sens. 2018, 10, 809. https://doi.org/10.3390/rs10060809
Hassan MA, Yang M, Rasheed A, Jin X, Xia X, Xiao Y, He Z. Time-Series Multispectral Indices from Unmanned Aerial Vehicle Imagery Reveal Senescence Rate in Bread Wheat. Remote Sensing. 2018; 10(6):809. https://doi.org/10.3390/rs10060809
Chicago/Turabian StyleHassan, Muhammad Adeel, Mengjiao Yang, Awais Rasheed, Xiuliang Jin, Xianchun Xia, Yonggui Xiao, and Zhonghu He. 2018. "Time-Series Multispectral Indices from Unmanned Aerial Vehicle Imagery Reveal Senescence Rate in Bread Wheat" Remote Sensing 10, no. 6: 809. https://doi.org/10.3390/rs10060809
APA StyleHassan, M. A., Yang, M., Rasheed, A., Jin, X., Xia, X., Xiao, Y., & He, Z. (2018). Time-Series Multispectral Indices from Unmanned Aerial Vehicle Imagery Reveal Senescence Rate in Bread Wheat. Remote Sensing, 10(6), 809. https://doi.org/10.3390/rs10060809