Evaluation of Chlorophyll-a and POC MODIS Aqua Products in the Southern Ocean
Abstract
:1. Introduction
2. Material and Methods
2.1. In Situ Dataset
Sample Collection and Storage
2.2. Satellite Data
2.3. Statistical Metrics
3. Results
4. Discussion
4.1. Satellite Versus In Situ Chlorophyll-a Comparison
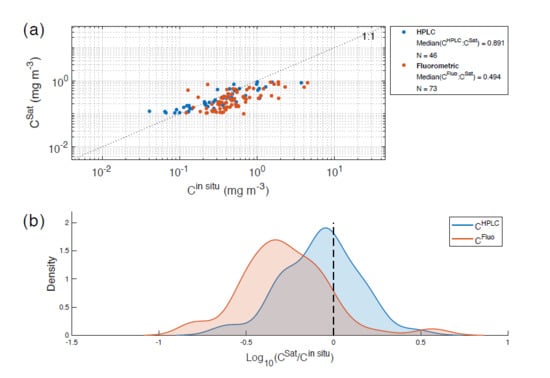
4.2. Comparison of HPLC and the Fluorometric Chlorophyll-a Methods
4.3. C vs. C
4.4. Satellite Versus In Situ POC Comparison
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APFZ | Antarctic Polar Front Zone |
| C | Chlorophyll-c concentration |
| C | Chlorophyll-a concentration measured by Fluorometry |
| C | Chlorophyll-a concentration measured by High Performance Liquid Chromatography |
| C | Chlorophyll-a concentration retrieved by satellite |
| CDOM | Colored Dissolved Organic Matter |
| CTD | Conductivity-Temperature-Depth |
| CV | Coefficient of Variation |
| DOC | Dissolved Organic Carbon |
| EOS | Earth Observing System |
| FSW | filtered seawater |
| HPLC | High Performance Liquid Chromatography |
| IOP | Inherent Optical Properties |
| JGOFS | Joint Global Ocean Flux Studies |
| MLD | Mixed Layer Depth |
| MODIS | MODerate resolution Imaging Spectroradiometer |
| MRAD | Mean Relative Absolute Difference |
| MRD | Mean Relative Difference |
| NASA | National Aeronautics and Space Administration |
| POC | Particulate Organic Carbon |
| POC | in situ concentration in Particulate Organic Carbon |
| POC | Particulate Organic Carbon concentration retrieved by satellite |
| RFU | Raw Fluorescence Units |
| RMSD | Root Mean Square Difference |
| SeaWIFS | Sea-viewing Wide Field-of-view Sensor |
| SO | Southern Ocean |
| SOCCOM | Southern Ocean Carbon and Climate Observations and Modeling |
| VIIRS | Visible Infrared Imager Radiometer Suite |
Appendix A
Appendix Algorithms Description
References
- Raven, J.A.; Falkowski, P.G. Oceanic sinks for atmospheric CO2. Plant Cell Environ. 1999, 22, 741–755. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.L.; Wallace, D.W.R.; Tilbrook, B.; et al. The oceanic sink for anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Khatiwala, S.; Primeau, F.; Hall, T. Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature 2009, 346–349, 554–577. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Sutherland, S.C.; Wanninkhof, R.; Sweeney, C.; Feely, R.A.; Chipman, D.W.; Hales, B.; Friederich, G.; Chavez, F.; Sabine, C. Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2009, 56, 554–577. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Sarmiento, J.L.; Paynter, D.J.; Dunne, J.P.; Krasting, J.P.; Winton, M. Dominance of the Southern Ocean in Anthropogenic Carbon and Heat Uptake in CMIP5 Models. J. Clim. 2015, 28, 862–886. [Google Scholar] [CrossRef]
- Sigman, D.M.; Boyle, E.A. Glacial/interglacial variations in atmospheric carbon dioxide. Nature 2000, 859–869, 554–577. [Google Scholar] [CrossRef]
- Pereira, E.S.; Garcia, C.A. Evaluation of satellite-derived MODIS chlorophyll algorithms in the northern Antarctic Peninsula. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2018, 149, 124–137. [Google Scholar] [CrossRef]
- Muller-Karger, F.; Varela, R.; Thunell, R.; Astor, Y.; Zhang, H.; Luerssen, R.; Hu, C. Processes of coastal upwelling and carbon flux in the Cariaco Basin. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2004, 51, 927–943. [Google Scholar] [CrossRef]
- Hu, C.; Muller-Karger, F.E.; Taylor, C.J.; Carder, K.L.; Kelble, C.; Johns, E.; Heil, C.A. Red tide detection and tracing using MODIS fluorescence data: A regional example in SW Florida coastal waters. Remote Sens. Environ. 2005, 97, 311–321. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Holm-Hansen, O. Bio-optical properties of Antarctic Peninsula waters: Differentiation from temperate ocean models. Deep Sea Res. A 1991, 38, 1009–1028. [Google Scholar] [CrossRef]
- Sullivan, C.W.; Arrigo, K.R.; McClain, C.R.; Comiso, J.C.; Firestone, J. Distributions of phytoplankton blooms in the Southern Ocean. Science 1993, 262, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, K.R.; Worthen, D.; Schnell, A.; Lizotte, M.P. Primary production in Southern Ocean waters. J. Geophys. Res. 2008, 103, 15587–15600. [Google Scholar] [CrossRef]
- Moore, J.K.; Abbott, M.R.; Richman, J.G.; Smith, W.O.; Cowles, T.J.; Coale, K.H.; Gardner, W.D.; Barber, R.T. SeaWiFS satellite ocean color data from the Southern Ocean. Geophys. Res. Lett. 1999, 26, 1465–1468. [Google Scholar] [CrossRef] [Green Version]
- Dierssen, H.M.; Smith, R.C. Bio-optical properties and remote sensing ocean color algorithms for Antarctic Peninsula waters. J. Geophys. Res. Oceans 2000, 105, 26301–26312. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Stramski, D.; Mitchell, B.G. A chlorophyll-dependent semianalytical reflectance model derived from field measurements of absorption and backscattering coefficients within the Southern Ocean. J. Geophys. Res. Oceans 2001, 106, 7125–7138. [Google Scholar] [CrossRef]
- Clementson, L.A.; Parslow, J.S.; Turnbull, A.R.; McKenzie, D.C.; Rathbone, C.E. Optical properties of waters in the Australasian sector of the Southern Ocean. J. Geophys. Res. Oceans 2001, 106, 31611–31625. [Google Scholar] [CrossRef]
- Barbini, R.; Colao, F.; Fantoni, R.; Fiorani, L.; Palucci, A.; Artamonov, E.S.; Galli, M. Remotely sensed primary production in the western Ross Sea: Results of in situ tuned models. Remote Sens. Environ. 2003, 15, 77–84. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Kahru, M.; Hewes, C.D.; Kawaguchi, S.; Kameda, T.; Sushin, V.A.; Krasovski, I.; Priddle, J.; Korb, R.; Hewitt, R.P.; et al. Temporal and spatial distribution of chlorophyll-a in surface waters of the Scotia Sea as determined by both shipboard measurements and satellite data. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2004, 51, 1323–1331. [Google Scholar] [CrossRef]
- Garcia, C.A.E.; Garcia, V.M.T.; McClain, C.R. Evaluation of SeaWiFS chlorophyll algorithms in the Southwestern Atlantic and Southern Oceans. Remote Sens. Environ. 2005, 95, 125–137. [Google Scholar] [CrossRef]
- Gregg, W.W.; Casey, N.W. Global and regional evaluation of the SeaWiFS chlorophyll data set. Remote Sens. Environ. 2004, 93, 463–479. [Google Scholar] [CrossRef]
- Korb, R.E.; Whitehouse, M.J.; Ward, P. SeaWiFS in the southern ocean: Spatial and temporal variability in phytoplankton biomass around South Georgia. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2004, 51, 99–116. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Kahru, M. Bio-optical algorithms for ADEOS-2 GLI. J. Remote Sens. Soc. Jpn. 2009, 29, 80–85. [Google Scholar] [CrossRef]
- Kahru, M.; Mitchell, B.G. Blending of ocean colour algorithms applied to the Southern Ocean. Remote Sens. Lett. 2010, 1, 119–124. [Google Scholar] [CrossRef]
- Szeto, M.; Werdell, P.J.; Moore, T.S.; Campbell, J.W. Are the world’s oceans optically different? J. Geophys. Res. Oceans 2011, 116, 1–14. [Google Scholar] [CrossRef]
- Guinet, C.; Xing, X.; Walker, E.; Monestiez, P.; Marchand, S.; Picard, B.; Jaud, T.; Authier, M.; Cotté, C.; Dragon, A.; et al. Calibration procedures and first data set of Southern Ocean chlorophyll a profiles collected by elephant seals equipped with a newly developed CTD-fluorescence tags. Earth Syst. Sci. Data 2013, 5, 15–29. [Google Scholar] [CrossRef]
- Johnson, R.; Strutton, P.G.; Wright, S.W.; McMinn, A.; Meiners, K.M. Three improved satellite chlorophyll algorithms for the Southern Ocean. J. Geophys. Res. Oceans 2013, 118, 3694–3703. [Google Scholar] [CrossRef]
- Dierssen, H.M. Perspectives on empirical approaches for ocean color remote sensing of chlorophyll in a changing climate. Proc. Natl. Acad. Sci. USA 2010, 107, 17073–17078. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.W.; Werdell, P.J. A multi-sensor approach for the on-orbit validation of ocean color satellite data products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- Haëntjens, N.; Boss, E.; Talley, L.D. Revisiting Ocean Color algorithms for chlorophyll a and particulate organic carbon in the Southern Ocean using biogeochemical floats. J. Geophys. Res. Oceans 2017, 122, 6583–6593. [Google Scholar] [CrossRef]
- Marrari, M.; Hu, C.; Daly, K. Validation of SeaWiFS chlorophyll a concentrations in the Southern Ocean: A revisit. Remote Sens. Environ. 2006, 105, 367–375. [Google Scholar] [CrossRef]
- Gibbs, C.F. Chlorophyll b interference in the fluorometric determination of chlorophyll a and ’phaeo-pigments’. Mar. Freshw. Res. 1979, 30, 597–606. [Google Scholar] [CrossRef]
- Welschmeyer, N.A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanog. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Ras, J.; Claustre, H.; Uitz, J. Spatial variability of phytoplankton pigment distributions in the Subtropical South Pacific Ocean: Comparison between in situ and predicted data. Biogeosciences 2008, 5, 353–369. [Google Scholar] [CrossRef]
- Knap, A.H.; Michaels, A.; Close, A.R.; Ducklow, H.; Dickson, A.G. Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurements; Reprint of Intergovernmental Oceanographic Commission Manuals and Guides, No. 29; UNESCO: Paris, France, 1994; p. 170. [Google Scholar]
- Ocean Color Feature. Available online: http://oceancolor.gsfc.nasa.gov (accessed on 4 July 2019).
- NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group. Moderate-resolution Imaging Spectroradiometer (MODIS) Aqua Ocean Color Data; 2018 Reprocessing; NASA OB.DAAC: Greenbelt, MD, USA, 2018. [CrossRef]
- Level 2 Ocean Color Flags. Available online: https://oceancolor.gsfc.nasa.gov/atbd/ocl2flags/ (accessed on 4 July 2019).
- Zheng, G.; DiGiacomo, P.M. Uncertainties and applications of satellite-derived coastal water quality products. Prog. Oceanogr. 2017, 159, 45–72. [Google Scholar] [CrossRef]
- Campbell, J.W. The lognormal distribution as a model for bio-optical variability in the sea. J. Geophys. Res. Oceans 1995, 100, 13237–13254. [Google Scholar] [CrossRef]
- Campbell, J.W.; O’Reilly, J.E. Metrics for Quantifying the Uncertainty in a Chlorophyll Algorithm: Explicit equations and examples using the OC4. v4 algorithm and NOMAD data. In Proceedings of the Ocean Color Bio-Optical Algorithm Mini (OCBAM) Workshop, New England Center, Southborough, MA, USA, 27–29 September 2005; pp. 1–15. [Google Scholar]
- Seegers, B.N.; Stumpf, R.P.; Schaeffer, B.A.; Loftin, K.A.; Werdell, P.J. Performance metrics for the assessment of satellite data products: An ocean color case study. Opt. Express 2018, 26, 7404–7422. [Google Scholar] [CrossRef] [PubMed]
- Ricker, W.E. Linear regressions in fishery research. J. Fish. Res. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Stramski, D.; Reynolds, R.A.; Kahru, M.; Mitchell, B.G. Estimation of particulate organic carbon in the ocean from satellite remote sensing. Sciences 1999, 285, 239–242. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll a algorithms for oligotrophic oceans: A novel approach based on three-band reflectance difference. J. Geophys. Res. Oceans 2012, 117. [Google Scholar] [CrossRef]
- Evers-King, H.; Martinez-Vicente, V.; Brewin, R.J.W.; Dall’Olmo, G.; Hickman, A.E.; Jackson, T.; Kostadinov, T.S.; Krasemann, H.; Loisel, H.; Röttgers, R.; et al. Validation and intercomparison of ocean color algorithms for estimating particulate organic carbon in the oceans. Front. Mar. Sci. 2017, 4, 1–19. [Google Scholar] [CrossRef]
- Cullen, J.J. The deep chlorophyll maximum: Comparing vertical profiles of chlorophyll a. Can. J. Fish. Aquat. Sci. 1982, 39, 791–803. [Google Scholar] [CrossRef]
- Proctor, C.W.; Roesler, C.S. New insights on obtaining phytoplankton concentration and composition from in situ multispectral Chlorophyll fluorescence. Limnol. Oceanogr. Methods 2010, 8, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Roesler, C.; Uitz, J.; Claustre, H.; Boss, E.; Xing, X.; Organelli, E.; Briggs, N.; Bricaud, A.; Schmechtig, C.; Poteau, A.; et al. Recommendations for obtaining unbiased chlorophyll estimates from in situ chlorophyll fluorometers: A global analysis of WET Labs ECO sensors. Limnol. Oceanogr. Methods 2017, 15, 572–585. [Google Scholar] [CrossRef]
- Kumari, B. Comparison of high performance liquid chromatography and fluorometric ocean colour pigments. J. Indian Soc. Remote 2005, 33, 541–546. [Google Scholar] [CrossRef]
- Kahru, M.; Mitchell, B.G. Chlorophyll a fluorescence in marine centric diatoms: Responses of chloroplasts to light and nutrient stress. Mar. Biol. 1973, 23, 39–46. [Google Scholar] [CrossRef]
- Morel, A.; Bricaud, A. Theoretical results concerning light absorption in a discrete medium, and application to specific absorption of phytoplankton. Deep Sea Res. A 1981, 28, 1375–1393. [Google Scholar] [CrossRef]
- Bricaud, A.; Morel, A.; Prieur, L. Optical efficiency factors of some phytoplankters. Limnol. Oceanogr. 1983, 28, 816–832. [Google Scholar] [CrossRef]
- Boss, E.; Swift, D.; Taylor, L.; Brickley, P.; Zaneveld, R.; Riser, S.; Perry, M.J.; Strutton, P.G. Observations of pigment and particle distributions in the western North Atlantic from an autonomous float and ocean color satellite. Limnol. Oceanogr. 2008, 53, 2112–2122. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, C.J. Chlorophyll b in the eastern North Pacific Ocean. Deep Sea Res. A 1981, 28, 1049–1056. [Google Scholar] [CrossRef]
- Trees, C.C.; Kennicutt, M.C., II; Brooks, J.M. Errors associated with the standard fluorimetric determination of chlorophylls and phaeopigments. Mar. Chem. 1985, 17, 1–12. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Lambert, C.; Biggs, D.C. Distribution of chlorophyll a and phaeopigments in the northwestern Gulf of Mexico: A comparison between fluorometric and high-performance liquid chromatography measurements. Bull. Mar. Sci. 1995, 56, 25–32. [Google Scholar]
- Dos Santos, A.C.A.; Calijuri, M.D.C.; Moraes, E.M.; Adorno, M.A.T.; Falco, P.B.; Carvalho, D.P.; Deberdt, G.L.B.; Benassi, S.F. Comparison of three methods for Chlorophyll determination: Spectrophotometry and Fluorimetry in samples containing pigment mixtures and spectrophotometry in samples with separate pigments through High Performance Liquid Chromatography. Acta Limnol. Bras. 2003, 15, 7–18. [Google Scholar]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D. Fluorometric determination of chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Jeffrey, S.W. A report of green algal pigments in the central North Pacific Ocean. Mar. Biol. 1976, 37, 33–37. [Google Scholar] [CrossRef]
- Bidigare, R.R.; Frank, T.J.; Zastrow, C.; Brooks, J.M. The distribution of algal chlorophylls and their degradation products in the Southern Ocean. Deep Sea Res. A 1986, 33, 923–937. [Google Scholar] [CrossRef]
- Parsons, T.R.; Takahashi, M.; Hargrave, B. Biological Oceanographic Processes, 3rd ed.; Oxford Pergamon Press: Oxford, UK, 1984; pp. 40–50. ISBN 0-08-030766-3. [Google Scholar]
- Arrigo, K.R.; Mills, M.M.; Kropuenske, L.R.; van Dijken, G.L.; Alderkamp, A.C.; Robinson, D.H. Photophysiology in two major Southern Ocean phytoplankton taxa: Photosynthesis and growth of Phaeocystis antarctica and Fragilariopsis cylindrus under different irradiance levels. Integr. Comp. Biol. 2010, 50, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.B.; de Souza, M.S.; Garcia, V.M.T.; Leal, M.C.; Brotas, V.; Garcia, C.A.E. Dynamics of phytoplankton communities during late summer around the tip of the Antarctic Peninsula. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2012, 65, 1–14. [Google Scholar] [CrossRef]
- Papagiannakis, E.; van Stokkum, I.H.M.; Fey, H.; Buchel, C.; van Grondelle, R. Spectroscopic characterization of the excitation energy transfer in the fucoxanthin–chlorophyll protein of diatoms. Photosynth. Res. 2005, 86, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Vaulot, D.; Birrien, J.L.; Marie, D.; Casotti, R.; Veldhuis, M.J.W.; Kraay, G.W.; Chrétiennot-Dinet, M.J. Morphology, ploidy, pigment composition, and genome size of cultured strains of Phaeocystis (Prymnesiophycea). J. Phycol. 1994, 30, 1022–1035. [Google Scholar] [CrossRef]
- Crosta, X.; Romero, O.; Armand, L.K.; Pichon, J.J. The biogeography of major diatom taxa in Southern Ocean sediments: 2. Open ocean related species. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 223, 66–92. [Google Scholar] [CrossRef]
- Boyd, P.W. Environmental factors controlling phytoplankton processes in the Southern Ocean. J. Phycol. 2002, 38, 844–861. [Google Scholar] [CrossRef]
- Coale, K.H.; Johnson, K.S.; Chavez, F.P.; Buesseler, K.O.; Barber, R.T.; Brzezinski, M.A.; Cochlan, W.P.M.; Millero, F.J.; Falkowski, P.G.; Bauer, J.E.; et al. Southern Ocean iron enrichment experiment: Carbon cycling in high-and low-Si waters. Science 2004, 304, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Swart, S.; Speich, S. An altimetry-based gravest empirical mode south of Africa: 2. Dynamic nature of the Antarctic Circumpolar Current fronts. J. Geophys. Res. Oceans 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Trull, T.W.; Bray, S.G.; Manganini, S.J.; Honjo, S.; François, R. Moored sediment trap measurements of carbon export in the Subantarctic and Polar Frontal zones of the Southern Ocean, south of Australia. J. Geophys. Res. Oceans 2001, 106, 31489–31509. [Google Scholar] [CrossRef] [Green Version]
- Lindenschmidt, K.E.; Chorus, I. The effect of water column mixing on phytoplankton succession, diversity and similarity. J. Plankton Res. 1998, 20, 1927–1951. [Google Scholar] [CrossRef]
- Stramski, D.; Reynolds, R.A.; Babin, M.; Kaczmarek, S.; Lewis, M.R.; Röttgers, R.; Sciandra, A.; Stramska, M.; Twardowski, M.S.; Franz, B.A.; et al. Relationships between the surface concentration of particulate organic carbon and optical properties in the eastern South Pacific and eastern Atlantic Oceans. Biogeosciences 2008, 5, 171–201. [Google Scholar] [CrossRef] [Green Version]
- Cetinić, I.; Perry, M.J.; Briggs, N.T.; Kallin, E.; D’Asaro, E.A.; Lee, C.M. Particulate organic carbon and inherent optical properties during 2008 North Atlantic Bloom Experiment. J. Geophys. Res. Oceans 2012, 117, 13237–13254. [Google Scholar] [CrossRef]
- Gardner, W.D.; Richardson, M.J.; Carlson, C.A.; Hansell, D.; Mishonov, A.V. Determining true particulate organic carbon: Bottles, pumps and methodologies. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2003, 50, 655–674. [Google Scholar] [CrossRef]





| Name | Date | |||
|---|---|---|---|---|
| SANAE 48 | December 2008–March 2009 | 0 | 110 | 198 |
| SANAE 49 | December 2009–February 2010 | 0 | 8 | 254 |
| Winter 12 | July 2012–August 2012 | 73 | 88 | 90 |
| Expedition | January 2013–February 2013 | 0 | 117 | 117 |
| SOSCEx 1 | February 2013–March 2013 | 97 | 95 | 129 |
| SANAE 53 | November 2013–February 2014 | 152 | 147 | 152 |
| Winter 15 | July 2015–August 2015 | 76 | 80 | 83 |
| SANAE 55 | December 2015–February 2015 | 147 | 172 | 175 |
| Winter 16 | July 2016–July 2016 | 63 | 0 | 0 |
| SANAE 56 | December 2016–February 2017 | 100 | 0 | 0 |
| ACE | December 2016–March 2017 | 320 | 193 | 329 |
| All | December 2008–March 2017 | 1028 | 1010 | 1527 |
| Box | Parameter | C vs. C | C vs. C | POC vs. POC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Box 1 | Time windows (hrs) | 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 |
| N | 27 | 41 | 59 | 73 | 20 | 29 | 39 | 46 | 16 | 25 | 38 | 46 | |
| Ratio | 0.53 | 0.47 | 0.49 | 0.49 | 0.78 | 0.86 | 0.91 | 0.89 | 0.65 | 0.69 | 0.74 | 0.74 | |
| MRD (%) | −33 | −37 | −38 | −35 | −8.5 | −3.5 | −2.2 | −5.8 | −32 | −24 | −23 | −22 | |
| MedRD (%) | −46.5 | −52.5 | −51.1 | −50.6 | −21.8 | −14.4 | −9.1 | −10.9 | −35 | −31 | −26 | −26 | |
| MARD (%) | 58 | 57 | 53 | 54 | 43.5 | 40.8 | 37 | 36.2 | 32 | 32 | 32 | 32 | |
| MedRAD (%) | 52.5 | 53.1 | 52.5 | 52.5 | 33.9 | 34.1 | 33.7 | 31.2 | 35 | 35 | 31 | 32 | |
| Bias_log | 0.52 | 0.52 | 0.52 | 0.53 | 0.78 | 0.84 | 0.87 | 0.84 | 0.67 | 0.72 | 0.73 | 0.74 | |
| MAD_log | 2.16 | 2.17 | 2.1 | 2.1 | 1.63 | 1.56 | 1.49 | 1.5 | 1.51 | 1.48 | 1.47 | 1.46 | |
| Median [in situ] | 0.47 | 0.45 | 0.47 | 0.45 | 0.36 | 0.31 | 0.29 | 0.33 | 118 | 108 | 81 | 89 | |
| Mean absolute | 1.8 ± | 2.9 ± | 4.3 ± | 5.6 ± | 1.9 ± | 2.8 ± | 3.9 ± | 4.9 ± | 1.8 ± | 2.9 ± | 4.6 ± | 5.6 ± | |
| time difference | 0.9 | 1.8 | 2.6 | 3.5 | 0.9 | 1.7 | 2.5 | 3.4 | 1.1 | 1.8 | 2.8 | 3.5 | |
| Box 2 | Time windows (hrs) | 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 |
| N | 36 | 54 | 74 | 91 | 27 | 40 | 52 | 63 | 25 | 38 | 55 | 66 | |
| Ratio | 0.48 | 0.47 | 0.48 | 0.48 | 0.83 | 0.82 | 0.87 | 0.85 | 0.68 | 0.72 | 0.73 | 0.74 | |
| MRD (%) | −38 | −42 | −42 | −39 | −9.8 | −10.6 | −8.4 | −10 | −31 | −23 | −22 | −19 | |
| MedRD (%) | −52.2 | −52.6 | −52 | −52 | −17.5 | −18.1 | −13.2 | −14.5 | −32 | −28 | −27 | −26 | |
| MARD (%) | 57 | 57 | 54 | 54 | 35.4 | 37.5 | 35.1 | 34.3 | 31 | 33 | 32 | 31 | |
| MedRAD (%) | 52.6 | 55.7 | 52.6 | 52.5 | 26.4 | 30.5 | 30 | 29.6 | 32 | 32 | 29 | 30 | |
| Bias_log | 0.49 | 0.47 | 0.48 | 0.5 | 0.8 | 0.79 | 0.82 | 0.81 | 0.68 | 0.73 | 0.74 | 0.76 | |
| MAD_log | 2.27 | 2.32 | 2.22 | 2.18 | 1.49 | 1.54 | 1.49 | 1.48 | 1.47 | 1.47 | 1.45 | 1.43 | |
| Median [in situ] | 0.44 | 0.44 | 0.46 | 0.44 | 0.31 | 0.34 | 0.32 | 0.33 | 115 | 109 | 90 | 89 | |
| Mean absolute | 1.6 ± | 2.7 ± | 3.9 ± | 5.2 ± | 1.7 ± | 2.6 ± | 3.6 ± | 4.9 ± | 1.6 ± | 2.6 ± | 4.2 ± | 5.3 ± | |
| time difference | 1 | 1.8 | 2.6 | 3.6 | 1 | 1.7 | 2.5 | 3.5 | 1.1 | 1.8 | 2.8 | 3.6 | |
| Parameter | Fluo | HPLC | POC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | 0.2< | ≥0.2 | All | 0.2< | ≥0.2 | All | [0,67[ | [67,124[ | ≥124 | All |
| N | 5 | 68 | 73 | 13 | 33 | 46 | 10 | 24 | 12 | 46 |
| Ratio | 1.04 | 0.48 | 0.49 | 1.34 | 0.8 | 0.9 | 0.93 | 0.67 | 0.65 | 0.74 |
| MRD (%) | 68.2 | −42.6 | −35 | 39.1 | −23.4 | −5.6 | 13 | −30 | −33 | −22 |
| MedRD (%) | 4.1 | −52.5 | −50.6 | 33.6 | −10.9 | −6.6 | −33.4 | −35.4 | −26 | |
| MARD (%) | 87.6 | 51 | 53.5 | 42.6 | 33.7 | 36.2 | 33 | 31 | 33 | 32 |
| MedRAD (%) | 36.7 | 52.6 | 52.5 | 33.6 | 28.7 | 31.2 | 25.3 | 33.4 | 35.4 | 31.9 |
| Bias_log | 1.34 | 0.49 | 0.53 | 1.32 | 0.7 | 0.84 | 1.06 | 0.68 | 0.65 | 0.74 |
| MAD_log | 1.69 | 2.13 | 2.1 | 1.37 | 1.55 | 1.5 | 2.14 | 1.35 | 1.47 | 1.54 |
| Median [in situ] | 0.15 | 0.47 | 0.45 | 0.11 | 0.44 | 0.33 | 51 | 85 | 159 | 89 |
| Expedition | N | a ± a | b ± b | R | RMSD | MRD | |
|---|---|---|---|---|---|---|---|
| SANAE 48 | 107 | 1.1 ± 0.06 | 0.09 ± 0.05 | 0.8 | 0.35 | 1.1 | 26 |
| SANAE 49 | 8 | 2.02 ± 0 | −0.12 ± 0 | 0.9 | 0.69 | 1.8 | 74 |
| Winter 12 | 88 | 0.85 ± 0.16 | 0.12 ± 0.04 | 0.2 | 0.16 | 1.3 | 39 |
| Expedition | 117 | 1.39 ± 0.07 | 0.02 ± 0.04 | 0.8 | 0.38 | 1.4 | 47 |
| SOSCEx 1 | 75 | 1.07 ± 0.1 | 0.02 ± 0.03 | 0.6 | 0.12 | 1.2 | 8 |
| SANAE 53 | 142 | 0.7 ± 0.08 | 0.16 ± 0.05 | 0.3 | 0.36 | 1.2 | 8 |
| Winter 15 | 80 | 1.48 ± 0.13 | 0 ± 0.04 | 0.6 | 0.16 | 1.4 | 47 |
| SANAE 55 | 172 | 1.99 ± 0.05 | −0.04 ± 0.04 | 0.9 | 0.77 | 1.9 | 91 |
| ACE | 192 | 2.21 ± 0.08 | 0.23 ± 0.05 | 0.8 | 1.15 | 2.8 | 283 |
| All | 981 | 1.66 ± 0.04 | 0 | 0.7 | 0.65 | 1.5 | 90 |
| Latitude Range | N | a ±a | b ±b | R | RMSD | MRD | C(mg m) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| [−80,−70 | 28 | 2.4 ± 0.32 | 0.06 ± 0.38 | 0.68 | 2.03 | 2.13 | 146 | 0.41 | 0.47 | 0.87 |
| [−70,−60 | 196 | 1.47 ± 0.08 | 0.12 ± 0.06 | 0.64 | 0.7 | 1.69 | 144 | 0.31 | 0.44 | 0.37 |
| [−60,−50 | 320 | 1.52 ± 0.06 | 0.07 ± 0.04 | 0.66 | 0.7 | 1.62 | 104 | 0.29 | 0.42 | 0.32 |
| [−50,−40 | 317 | 1.6 ± 0.06 | −0.04 ± 0.03 | 0.67 | 0.34 | 1.38 | 54 | 0.25 | 0.11 | 0.3 |
| [−40,−30 | 120 | 1.15 ± 0.08 | 0.08 ± 0.03 | 0.6 | 0.2 | 1.38 | 45 | 0.17 | 0.07 | 0.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moutier, W.; Thomalla, S.J.; Bernard, S.; Wind, G.; Ryan-Keogh, T.J.; Smith, M.E. Evaluation of Chlorophyll-a and POC MODIS Aqua Products in the Southern Ocean. Remote Sens. 2019, 11, 1793. https://doi.org/10.3390/rs11151793
Moutier W, Thomalla SJ, Bernard S, Wind G, Ryan-Keogh TJ, Smith ME. Evaluation of Chlorophyll-a and POC MODIS Aqua Products in the Southern Ocean. Remote Sensing. 2019; 11(15):1793. https://doi.org/10.3390/rs11151793
Chicago/Turabian StyleMoutier, William, Sandy J Thomalla, Stewart Bernard, Galina Wind, Thomas J Ryan-Keogh, and Marié E Smith. 2019. "Evaluation of Chlorophyll-a and POC MODIS Aqua Products in the Southern Ocean" Remote Sensing 11, no. 15: 1793. https://doi.org/10.3390/rs11151793
APA StyleMoutier, W., Thomalla, S. J., Bernard, S., Wind, G., Ryan-Keogh, T. J., & Smith, M. E. (2019). Evaluation of Chlorophyll-a and POC MODIS Aqua Products in the Southern Ocean. Remote Sensing, 11(15), 1793. https://doi.org/10.3390/rs11151793