Toward Quantifying Oil Contamination in Vegetated Areas Using Very High Spatial and Spectral Resolution Imagery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Hyperspectral Data Acquisition and Preprocessing
2.2.1. Airborne Images
2.2.2. Field Reflectance
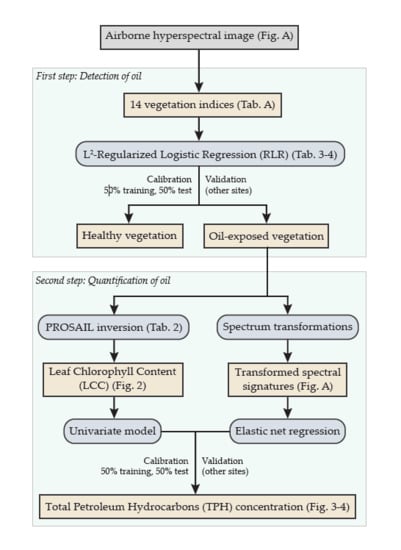
2.3. First Step of the Approach: Detection of Oil Contamination
2.4. Second Step of the Approach: Quantification of Soil TPH Content
2.4.1. First Method Based on PROSPECT and PROSAIL
2.4.2. Second Method Based on Elastic Net Regression
3. Results
3.1. Calibration of the Methods
3.1.1. Detection of Oil Contamination
3.1.2. Quantification of Soil TPH Content
PROSAIL-Based Method
Elastic Net Based Method
3.2. Validation of the Methods
3.2.1. Validation of Oil Detection
3.2.2. Validation of TPH Quantification
4. Discussion and Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Index | Formula | Reference | Related Farameter |
|---|---|---|---|
| Chlorophyll/Carotenoids Index | [98] | Chl, B-car | |
| Carter Index 2 | [99] | Chl, Lut | |
| Gitelson & Merzlyak Index 1 | [77] | Chl, B-car | |
| Gitelson & Merzlyak Index 2 | [77] | Chl, B-car | |
| modified Simple Ratio 705 nm | [26] | Chl, B-car | |
| MERIS Terrestrial Chlorophyll Index | [76] | Chl, B-car | |
| Normalized Difference 705 nm | [26] | Chl, B-car | |
| Photochemical Reflectance Index 2 | [100] | Chl, B-car | |
| Photochemical Reflectance Index 3 | [100] | Lut, B-car | |
| Structure Intensive Pigment Index 2 | [73] | Lut, Ant | |
| Simple Ratio 705 nm | [26] | Chl, B-car | |
| Vogelmann Index 2 | [78] | Lut | |
| Vogelmann Index 3 | [78] | Lut | |
| Disease Water Stress Index | [101] | LWC |


References
- Miller, R.G.; Sorrell, S.R.; Lane, H.; Kt, S. The future of oil supply. Philos. Trans. R. Soc. A 2014, 372, 20130179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrell, S.; Miller, R.; Bentley, R.; Speirs, J. Oil futures: A comparison of global supply forecasts. Energy Policy 2010, 38, 4990–5003. [Google Scholar] [CrossRef]
- Barraza, F.; Maurice, L.; Uzu, G.; Becerra, S.; López, F.; Ochoa-Herrera, V.; Ruales, J.; Schreck, E. Distribution, contents and health risk assessment of metal(loid)s in small-scale farms in the Ecuadorian Amazon: An insight into impacts of oil activities. Sci. Total Environ. 2018, 622–623, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Karki, H. Application of robotics in onshore oil and gas industry—A review Part I. Rob. Auton. Syst. 2016, 75, 490–507. [Google Scholar] [CrossRef]
- Slonecker, T.; Fisher, G.B.; Aiello, D.P.; Haack, B. Visible and infrared remote imaging of hazardous waste: A review. Remote Sens. 2010, 2, 2474–2508. [Google Scholar] [CrossRef]
- Chang, J.I.; Lin, C.C. A study of storage tank accidents. J. Loss Prev. Process Ind. 2006, 19, 51–59. [Google Scholar] [CrossRef]
- Shadizadeh, S.R.; Zoveidavianpoor, M. A drilling reserve mud pit assessment in Iran: Environmental impacts and awareness. Pet. Sci. Technol. 2010, 28, 1513–1526. [Google Scholar] [CrossRef]
- Van der Werff, H.; van der Meijde, M.; Jansma, F.; van der Meer, F.; Groothuis, G.J. A Spatial-Spectral Approach for Visualization of Vegetation Stress Resulting from Pipeline Leakage. Sensors 2008, 8, 3733–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, X.; Wang, B.; Lu, Q. Fragmentation effects of oil wells and roads on the Yellow River Delta, North China. Ocean Coast. Manag. 2011, 54, 256–264. [Google Scholar] [CrossRef]
- Finer, M.; Jenkins, C.N.; Pimm, S.L.; Keane, B.; Ross, C. Oil and gas projects in the Western Amazon: Threats to wilderness, biodiversity, and indigenous peoples. PLoS ONE 2008, 3, e2932. [Google Scholar] [CrossRef]
- Jones, N.F.; Pejchar, L.; Kiesecker, J.M. The energy footprint: How oil, natural gas, and wind energy affect land for biodiversity and the flow of ecosystem services. Bioscience 2015, 65, 290–301. [Google Scholar] [CrossRef]
- Onyia, N.; Balzter, H.; Berrio, J.-C. Normalized Difference Vegetation Vigour Index: A New Remote Sensing Approach to Biodiversity Monitoring in Oil Polluted Regions. Remote Sens. 2018, 10, 897. [Google Scholar] [CrossRef]
- Kisic, I.; Mesic, S.; Basic, F.; Brkic, V.; Mesic, M.; Durn, G.; Zgorelec, Z.; Bertovic, L. The effect of drilling fluids and crude oil on some chemical characteristics of soil and crops. Geoderma 2009, 149, 209–216. [Google Scholar] [CrossRef]
- Wang, Z.; Fingas, M.; Page, D.S. Oil Spill Identification. J. Chromatogr. A 1999, 843, 369–411. [Google Scholar] [CrossRef]
- Correa Pabón, R.E.; de Souza Filho, C.R. Spectroscopic characterization of red latosols contaminated by petroleum-hydrocarbon and empirical model to estimate pollutant content and type. Remote Sens. Environ. 2016, 175, 323–336. [Google Scholar] [CrossRef]
- Scafutto, R.D.P.M.; de Souza Filho, C.R.; de Oliveira, W.J. Hyperspectral remote sensing detection of petroleum hydrocarbons in mixtures with mineral substrates: Implications for onshore exploration and monitoring. ISPRS J. Photogramm. Remote Sens. 2017, 128, 146–157. [Google Scholar] [CrossRef]
- Correa Pabón, R.E.; de Souza Filho, C.R.; Oliveira, W.J. de Reflectance and imaging spectroscopy applied to detection of petroleum hydrocarbon pollution in bare soils. Sci. Total Environ. 2019, 649, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Hese, S.; Schmullius, C. High spatial resolution image object classification for terrestrial oil spill contamination mapping in West Siberia. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 130–141. [Google Scholar] [CrossRef]
- Arellano, P.; Tansey, K.; Balzter, H.; Boyd, D.S. Detecting the effects of hydrocarbon pollution in the Amazon forest using hyperspectral satellite images. Environ. Pollut. 2015, 205, 225–239. [Google Scholar] [CrossRef]
- Emengini, E.J.; Blackburn, G.A.; Theobald, J.C. Early detection of oil-induced stress in crops using spectral and thermal responses. J. Appl. Remote Sens. 2013, 7, 73596. [Google Scholar] [CrossRef]
- Gürtler, S.; de Souza Filho, C.R.; Sanches, I.D.; Alves, M.N.; Oliveira, W.J. Determination of changes in leaf and canopy spectra of plants grown in soils contaminated with petroleum hydrocarbons. ISPRS J. Photogramm. Remote Sens. 2018, 146, 272–288. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Kopačková, V. Detecting vegetation stress as a soil contamination proxy: A review of optical proximal and remote sensing techniques. Int. J. Environ. Sci. Technol. 2019, 16, 2511–2524. [Google Scholar] [CrossRef]
- Baruah, P.; Saikia, R.R.; Baruah, P.P.; Deka, S. Effect of crude oil contamination on the chlorophyll content and morpho-anatomy of Cyperus brevifolius (Rottb.) Hassk. Environ. Sci. Pollut. Res. 2014, 21, 12530–12538. [Google Scholar] [CrossRef]
- Balliana, A.G.; Moura, B.B.; Inckot, R.C.; Bona, C. Development of Canavalia ensiformis in soil contaminated with diesel oil. Environ. Sci. Pollut. Res. 2017, 24, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Nakata, C.; Qualizza, C.; MacKinnon, M.; Renault, S. Growth and physiological responses of Triticum aestivum and Deschampsia caespitosa exposed to petroleum coke. Water. Air. Soil Pollut. 2011, 216, 59–72. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Ustin, S.L.; Schaepman, M.E.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Lassalle, G.; Fabre, S.; Credoz, A.; Hédacq, R.; Borderies, P.; Bertoni, G.; Erudel, T.; Buffan-Dubau, E.; Dubucq, D.; Elger, A. Detection and discrimination of various oil-contaminated soils using vegetation reflectance. Sci. Total Environ. 2019, 655, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Rosso, P.H.; Pushnik, J.C.; Lay, M.; Ustin, S.L. Reflectance properties and physiological responses of Salicornia virginica to heavy metal and petroleum contamination. Environ. Pollut. 2005, 137, 241–252. [Google Scholar] [CrossRef]
- Sanches, I.D.; de Souza Filho, C.R.; Magalhães, L.A.; Quitério, G.C.M.; Alves, M.N.; Oliveira, W.J. Assessing the impact of hydrocarbon leakages on vegetation using reflectance spectroscopy. ISPRS J. Photogramm. Remote Sens. 2013, 78, 85–101. [Google Scholar] [CrossRef]
- Adamu, B.; Tansey, K.; Ogutu, B. Remote sensing for detection and monitoring of vegetation affected by oil spills. Int. J. Remote Sens. 2018, 39, 3628–3645. [Google Scholar] [CrossRef] [Green Version]
- Ozigis, M.S.; Kaduk, J.D.; Jarvis, C.H. Mapping terrestrial oil spill impact using machine learning random forest and Landsat 8 OLI imagery: A case site within the Niger Delta region of Nigeria. Environ. Sci. Pollut. Res. 2019, 26, 3621–3635. [Google Scholar] [CrossRef] [PubMed]
- Arellano, P.; Tansey, K.; Balzter, H.; Tellkamp, M. Plant family-specific impacts of petroleum pollution on biodiversity and leaf chlorophyll content in the Amazon rainforest of Ecuador. PLoS ONE 2017, 12, e0169867. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, G.; Credoz, A.; Hédacq, R.; Fabre, S.; Dubucq, D.; Elger, A. Assessing Soil Contamination Due to Oil and Gas Production Using Vegetation Hyperspectral Reflectance. Environ. Sci. Technol. 2018, 52, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, S.; Wang, D.; Zhou, C.; van der Meer, F.; Zhang, Y. Hydrocarbon micro-seepage detection from airborne hyper-spectral images by plant stress spectra based on the PROSPECT model. Int. J. Appl. Earth Obs. Geoinf. 2019, 74, 180–190. [Google Scholar] [CrossRef]
- Credoz, A.; Hédacq, R.; Barreau, C.; Dubucq, D. Experimental study of hyperspectral responses of plants grown on mud pit soil. In Proceedings of the Earth Resources and Environmental Remote Sensing/GIS Applications VII, Edinburgh, UK, 27–29 September 2016; Volume 10005, p. 100051E. [Google Scholar]
- Emengini, E.J.; Blackburn, G.A.; Theobald, J.C. Discrimination of plant stress caused by oil pollution and waterlogging using hyperspectral and thermal remote sensing. J. Appl. Remote Sens. 2013, 7, 73476. [Google Scholar] [CrossRef]
- Lassalle, G.; Credoz, A.; Fabre, S.; Elger, A.; Hédacq, R.; Dubucq, D. Hyperspectral signature analysis of three plant species to long-term hydrocarbon and heavy metal exposure. In Proceedings of the Earth Resources and Environmental Remote Sensing/GIS Applications VIII, Warsaw, Poland, 11–14 September 2017; Michel, U., Schulz, K., Eds.; SPIE: Bellingham, WA, USA, 2017; Volume 10428, p. 33. [Google Scholar]
- Noomen, M.F.; Skidmore, A.K.; van der Meer, F.D.; Prins, H.H.T. Continuum removed band depth analysis for detecting the effects of natural gas, methane and ethane on maize reflectance. Remote Sens. Environ. 2006, 105, 262–270. [Google Scholar] [CrossRef]
- Sanches, I.D.; Souza Filho, C.R.; Magalhães, L.A.; Quitério, G.C.M.; Alves, M.N.; Oliveira, W.J. Unravelling remote sensing signatures of plants contaminated with gasoline and diesel: An approach using the red edge spectral feature. Environ. Pollut. 2013, 174, 16–27. [Google Scholar] [CrossRef]
- Arellano, P.; Tansey, K.; Balzter, H.; Boyd, D.S. Field spectroscopy and radiative transfer modelling to assess impacts of petroleum pollution on biophysical and biochemical parameters of the Amazon rainforest. Environ. Earth Sci. 2017, 76, 1–14. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Lassalle, G.; Fabre, S.; Credoz, A.; Hédacq, R.; Bertoni, G.; Dubucq, D.; Elger, A. Application of PROSPECT for estimating total petroleum hydrocarbons in contaminated soils from leaf optical properties. J. Hazard. Mater. 2019, 377, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Verhoef, W. Light scattering by leaf layers with application to canopy reflectance modeling: The SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT+SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Brigot, G.; Colin-Koeniguer, E.; Plyer, A.; Janez, F. Adaptation and Evaluation of an Optical Flow Method Applied to Coregistration of Forest Remote Sensing Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 2923–2939. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.M.; Milton, E.J. The use of the empirical line method to calibrate remotely sensed data to reflectance. Int. J. Remote Sens. 1999, 20, 2653–2662. [Google Scholar] [CrossRef]
- Roberts, D.A.; Smith, M.O.; Adams, J.B. Green vegetation, nonphotosynthetic vegetation, and soils in AVIRIS data. Remote Sens. Environ. 1993, 44, 255–269. [Google Scholar] [CrossRef]
- Erudel, T.; Fabre, S.; Houet, T.; Mazier, F.; Briottet, X. Criteria Comparison for Classifying Peatland Vegetation Types Using In Situ Hyperspectral Measurements. Remote Sens. 2017, 9, 748. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. In Technometrics; Palgrave Macmillan: London, UK, 1969; Volume 11, pp. 137–148. [Google Scholar]
- Story, M.; Congalton, R.G. Remote Sensing Brief Accuracy Assessment: A User’s Perspective. Photogramm. Eng. Remote Sens. 1986, 52, 397–399. [Google Scholar]
- Wei, C.; Huang, J.; Wang, X.; Blackburn, G.A.; Zhang, Y.; Wang, S.; Mansaray, L.R. Hyperspectral characterization of freezing injury and its biochemical impacts in oilseed rape leaves. Remote Sens. Environ. 2017, 195, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Feret, J.B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Féret, J.B.; Gitelson, A.A.; Noble, S.D.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Jacquemoud, S.; Baret, F.; Andrieu, B.; Danson, F.M.; Jaggard, K. Extraction of Vegetation Biophysical Parameters by Inversion of the PROSPECT + SAIL Models on Sugar Beet Canopy Reflectance Data. Application to TM and AVIRIS Sensors. Remote Sens. Environ. 1995, 52, 163–172. [Google Scholar] [CrossRef]
- Sun, J.; Shi, S.; Yang, J.; Du, L.; Gong, W.; Chen, B.; Song, S. Analyzing the performance of PROSPECT model inversion based on different spectral information for leaf biochemical properties retrieval. ISPRS J. Photogramm. Remote Sens. 2018, 135, 74–83. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C. Inversion of a radiative transfer model for estimating vegetation LAI and chlorophyll in a heterogeneous grassland. Remote Sens. Environ. 2008, 112, 2592–2604. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef]
- Storn, R.; Price, K. Differential Evolution—A Simple and Efficient Heuristic for Global Optimization over Continuous Spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T. Evaluation of the PROSAIL model capabilities for future hyperspectral model environments: A review study. Remote Sens. 2018, 10, 85. [Google Scholar] [CrossRef]
- Botha, E.J.; Leblon, B.; Zebarth, B.; Watmough, J. Non-destructive estimation of potato leaf chlorophyll from canopy hyperspectral reflectance using the inverted PROSAIL model. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 360–374. [Google Scholar] [CrossRef]
- Si, Y.; Schlerf, M.; Zurita-Milla, R.; Skidmore, A.; Wang, T. Mapping spatio-temporal variation of grassland quantity and quality using MERIS data and the PROSAIL model. Remote Sens. Environ. 2012, 121, 415–425. [Google Scholar] [CrossRef]
- Balandier, P.; Marquier, A.; Casella, E.; Kiewitt, A.; Coll, L.; Wehrlen, L.; Harmer, R. Architecture, cover and light interception by bramble (Rubus fruticosus): A common understorey weed in temperate forests. Forestry 2013, 86, 39–46. [Google Scholar] [CrossRef]
- Shi, T.; Liu, H.; Wang, J.; Chen, Y.; Fei, T.; Wu, G. Monitoring arsenic contamination in agricultural soils with reflectance spectroscopy of rice plants. Environ. Sci. Technol. 2014, 48, 6264–6272. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, T.; Skidmore, A.K.; Shi, T.; Wu, G. Evaluating different methods for grass nutrient estimation from canopy hyperspectral reflectance. Remote Sens. 2015, 7, 5901–5917. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, S.; Zhang, Y.; Zhao, J.; Song, D.; Liu, D. Evaluating Metal Effects on the Reflectance Spectra of Plant Leaves during Different Seasons in Post-Mining Areas, China. Remote Sens. 2018, 10, 1211. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regression and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, Y.; Yu, L.; Liu, Y.; Liu, Y.; Zhang, Y.; Liu, Y.; Cheng, H. Combining fractional order derivative and spectral variable selection for organic matter estimation of homogeneous soil samples by VIS-NIR spectroscopy. Remote Sens. 2018, 10, 479. [Google Scholar] [CrossRef]
- Prospere, K.; McLaren, K.; Wilson, B. Plant species discrimination in a tropical wetland using in situ hyperspectral data. Remote Sens. 2014, 6, 8494–8523. [Google Scholar] [CrossRef]
- Peng, X.; Shi, T.; Song, A.; Chen, Y.; Gao, W. Estimating soil organic carbon using VIS/NIR spectroscopy with SVMR and SPA methods. Remote Sens. 2014, 6, 2699–2717. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and carotenoids at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Verrelst, J.; Schaepman, M.E.; Koetz, B.; Kneubühler, M. Angular sensitivity analysis of vegetation indices derived from CHRIS/PROBA data. Remote Sens. Environ. 2008, 112, 2341–2353. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. Evaluation of the MERIS terrestrial chlorophyll index (MTCI). Adv. Space Res. 2007, 39, 100–104. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Nujkić, M.M.; Dimitrijević, M.M.; Alagić, S.Č.; Tošić, S.B.; Petrović, J.V. Impact of metallurgical activities on the content of trace elements in the spatial soil and plant parts of Rubus fruticosus L. Environ. Sci. Process. Impacts 2016, 18, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Athar, H.R.; Ambreen, S.; Javed, M.; Hina, M.; Rasul, S.; Zafar, Z.U.; Manzoor, H.; Ogbaga, C.C.; Afzal, M.; Al-Qurainy, F.; et al. Influence of sub-lethal crude oil concentration on growth, water relations and photosynthetic capacity of maize (Zea mays L.) plants. Environ. Sci. Pollut. Res. 2016, 23, 18320–18331. [Google Scholar] [CrossRef]
- Han, G.; Cui, B.X.; Zhang, X.X.; Li, K.R. The effects of petroleum-contaminated soil on photosynthesis of Amorpha fruticosa seedlings. Int. J. Environ. Sci. Technol. 2016, 13, 2383–2392. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.L.L.; Steven, M.D.D.; Colls, J.J.J. Use of hyperspectral derivative ratios in the red-edge region to identify plant stress responses to gas leaks. Remote Sens. Environ. 2004, 92, 207–217. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Z.; Wang, J.; Ding, J.; Yu, Y.; Li, J.; Xiao, N.; Jiang, L.; Zheng, Y.; Rimmington, G.M. Monitoring plant response to phenanthrene using the red edge of canopy hyperspectral reflectance. Mar. Pollut. Bull. 2014, 86, 332–341. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, F.; van Dijk, P.; van der Werff, H.; Yang, H. Remote sensing and petroleum seepage: A review and case study. Terra Nov. 2002, 14, 1–17. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: A comparison of indices based on liquid water and chlorophyll absorption features. Remote Sens. Environ. 2003, 84, 526–537. [Google Scholar] [CrossRef]
- Wang, Z.; Skidmore, A.K.; Wang, T.; Darvishzadeh, R.; Hearne, J. Applicability of the PROSPECT model for estimating protein and cellulose+lignin in fresh leaves. Remote Sens. Environ. 2015, 168, 205–218. [Google Scholar] [CrossRef]
- Emengini, E.J.; Ezeh, F.C.; Chigbu, N. Comparative Analysis of Spectral Responses of Varied Plant Species to Oil Stress. Int. J. Sci. Eng. Res. 2013, 4, 1421–1427. [Google Scholar]
- Noomen, M.F.; van der Werff, H.M.A.; van der Meer, F.D. Spectral and spatial indicators of botanical changes caused by long-term hydrocarbon seepage. Ecol. Inform. 2012, 8, 55–64. [Google Scholar] [CrossRef]
- Dorrington, V.H.; Pyatt, F.B. Some aspects of tissue accumulation and tolerance to available heavy metal ions by Rubus Fruticosus L., A Colonizer of spoil tips in S.W. England. Int. J. Environ. Stud. 1983, 20, 229–237. [Google Scholar] [CrossRef]
- Bioucas-Dias, J.M.; Plaza, A.; Dobigeon, N.; Parente, M.; Du, Q.; Gader, P.; Chanussot, J. Hyperspectral unmixing overview: Geometrical, statistical, and sparse regression-based approaches. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 354–379. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, L.; Zhang, L. An adaptive differential evolution endmember extraction algorithm for hyperspectral remote sensing imagery. IEEE Geosci. Remote Sens. Lett. 2014, 11, 1061–1065. [Google Scholar] [CrossRef]
- Stagakis, S.; Vanikiotis, T.; Sykioti, O. Estimating forest species abundance through linear unmixing of CHRIS/PROBA imagery. ISPRS J. Photogramm. Remote Sens. 2016, 119, 79–89. [Google Scholar] [CrossRef]
- Dehaan, R.; Louis, J.; Wilson, A.; Hall, A.; Rumbachs, R. Discrimination of blackberry (Rubus fruticosus sp. agg.) using hyperspectral imagery in Kosciuszko National Park, NSW, Australia. ISPRS J. Photogramm. Remote Sens. 2007, 62, 13–24. [Google Scholar] [CrossRef]
- Cunha, S.B. da A review of quantitative risk assessment of onshore pipelines. J. Loss Prev. Process Ind. 2016, 44, 282–298. [Google Scholar] [CrossRef]
- Asadzadeh, S.; de Souza Filho, C.R. Spectral remote sensing for onshore seepage characterization: A critical overview. Earth-Science Rev. 2017, 168, 48–72. [Google Scholar] [CrossRef] [Green Version]
- Sims, D.A.; Luo, H.; Hastings, S.; Oechel, W.C.; Rahman, A.F.; Gamon, J.A. Parallel adjustments in vegetation greenness and ecosystem CO2 exchange in response to drought in a Southern California chaparral ecosystem. Remote Sens. Environ. 2006, 103, 289–303. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 517–520. [Google Scholar] [CrossRef]
- Gamon, J.A.J.A.; Peñuelas, J.; Field, C.B.B.; Penuelas, J.; Field, C.B.B. a Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Detecting sugarcane ‘orange rust’ disease using EO-1 Hyperion hyperspectral imagery. Int. J. Remote Sens. 2004, 25, 489–498. [Google Scholar] [CrossRef]





| Step | Zone Type | Number of Zones (n) | Total Pixel Count | C10-C40 TPH (g/kg−1) |
|---|---|---|---|---|
| training + test | control | 1 | 388 | <DL |
| training + test | mud pit (“brownfield”) | 10 | 184 | 17–39 |
| validation | control | 5 | 211 | <DL |
| validation | mud pit | 4 | 33 | 0.25 |
| 20 | 0.38 | |||
| 48 | 3.15 | |||
| 22 | 24 |
| Parameter | Unit | Range | Reference |
|---|---|---|---|
| PROSPECT: | |||
| Leaf structure (N) | 1–5 | [43] | |
| Chlorophyll a + b (Cab) | µg/cm−2 | 1–100 | |
| Carotenoids (Ccx) | µg/cm−2 | 1–50 | |
| Brown pigments (Cbp) | µg/cm−2 | 0.01–1 | |
| Water (Cw) | g/cm−2 | 0.001–0.1 | |
| Dry matter (Cm) | g/cm−2 | 0.001–0.1 | |
| SAIL: | |||
| Leaf Area Index (LAI) | m2/m−2 | 0.1–5 | [59,60,62,63,64] |
| Hotspot (hot) | m/m−1 | 0.01–0.1 | |
| Leaf Inclination Distribution Function (LIDF) | Planophile 1 | ||
| Soil brightness 2 (bright) | 0.5–1.5 | ||
| Solar zenith angle (θs) | deg. | Fixed (30°) | |
| Viewing zenith angle (θv) | deg. | Fixed (0°) | |
| Relative azimuth angle (φsv) | deg. | Fixed (0°) |
| Brownfield | Control | User’s Accuracy (%) | |
|---|---|---|---|
| Brownfield | 88 | 4 | 95,7 |
| Control | 2 | 192 | 99.0 |
| Producer’s accuracy (%) | 97.8 | 98.0 |
| C10-C40 TPH (g/kg−1) | Mud Pit | Control | User’s Accuracy (%) | |
|---|---|---|---|---|
| Control 1 | <DL | 13 | 146 | 91.8 |
| Mud pit | 0.25 | 2 | 31 | 6.1 |
| 0.38 | 3 | 17 | 15 | |
| 3.15 | 41 | 7 | 85.4 | |
| 24 | 21 | 1 | 95.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lassalle, G.; Elger, A.; Credoz, A.; Hédacq, R.; Bertoni, G.; Dubucq, D.; Fabre, S. Toward Quantifying Oil Contamination in Vegetated Areas Using Very High Spatial and Spectral Resolution Imagery. Remote Sens. 2019, 11, 2241. https://doi.org/10.3390/rs11192241
Lassalle G, Elger A, Credoz A, Hédacq R, Bertoni G, Dubucq D, Fabre S. Toward Quantifying Oil Contamination in Vegetated Areas Using Very High Spatial and Spectral Resolution Imagery. Remote Sensing. 2019; 11(19):2241. https://doi.org/10.3390/rs11192241
Chicago/Turabian StyleLassalle, Guillaume, Arnaud Elger, Anthony Credoz, Rémy Hédacq, Georges Bertoni, Dominique Dubucq, and Sophie Fabre. 2019. "Toward Quantifying Oil Contamination in Vegetated Areas Using Very High Spatial and Spectral Resolution Imagery" Remote Sensing 11, no. 19: 2241. https://doi.org/10.3390/rs11192241
APA StyleLassalle, G., Elger, A., Credoz, A., Hédacq, R., Bertoni, G., Dubucq, D., & Fabre, S. (2019). Toward Quantifying Oil Contamination in Vegetated Areas Using Very High Spatial and Spectral Resolution Imagery. Remote Sensing, 11(19), 2241. https://doi.org/10.3390/rs11192241