UAV-Based High Resolution Thermal Imaging for Vegetation Monitoring, and Plant Phenotyping Using ICI 8640 P, FLIR Vue Pro R 640, and thermoMap Cameras
Abstract
:1. Introduction
2. UAVs and Thermal Cameras
2.1. ICI 8640 P
2.2. FLIR Vue Pro R 640
2.3. thermoMap
2.4. FLIR TG167
3. Test Sites and Case Studies
3.1. Case Study 1: Vegetation Monitoring in Forest Park, St. Louis, Missouri
3.2. Case Study 2: Plant Phenotyping and Early Stress Detection near Columbia, Missouri
3.2.1. Experimental Setup
3.2.2. Data Collection
3.3. Case Study 3: High throughput Phenotyping at the Maricopa Agricultural Center
3.3.1. Experimental Setup
3.3.2. Data Collection
4. Methods
4.1. UAV Image Pre-Processing
4.2. Thermal Image Calibration
4.3. Image Quality Assessment
4.4. Processing of Images to Remove Non-Vegetation Pixels
4.5. Statistical Analysis
4.5.1. ANOVA Test and Correlation Analysis
4.5.2. Heritability Analysis for Case Study 3
5. Results
5.1. Case Study 1: Vegetation Monitoring in Forest Park, St. Louis, Missouri
5.2. Case Study 2: Plant Phenotyping and Early Stress Detection near Columbia, Missouri
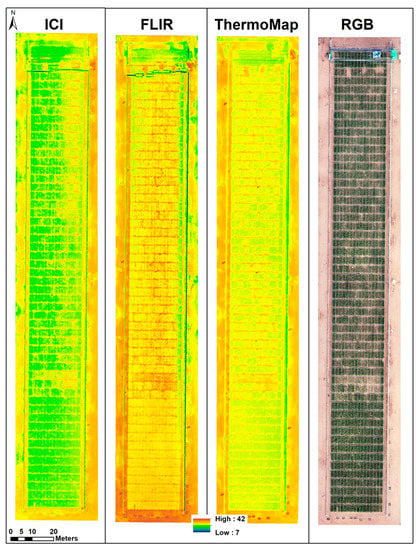
5.2.1. Visual Evaluation and Comparison
5.2.2. Temperature Accuracy Assessment
5.2.3. ANOVA Test for Different Soybean Genotypes and Rooting Depth Treatments
5.2.4. Correlation Analysis between Canopy Temperature and Plant Phenotypes
5.3. Case Study 3: High Throughput Phenotyping at Maricopa Agricultural Center
5.3.1. Visual Evaluation and Comparison
5.3.2. Heritability Analysis and Phenotype Estimation
5.4. Image Quality Assessment and Comparison
6. Discussion
6.1. Thermal Cameras for Plant Phenotyping
6.2. Water Stress Detection
6.3. Impact of Camera Focal Length and Ground Sampling Distance
6.4. Limitations of the Study
7. Conclusions
- The ICI and FLIR cameras provided good image quality. The ICI camera provided the best score in terms of Naturalness Image Quality Evaluator (NIQE), while FLIR yielded better Blur Metric (BM) and Vollath’s Correlation (VC) scores. The ICI provided a more consistent and visually appealing result than the FLIR, but, as indicated by the quality tests, both of the cameras are capable of providing different sets of high-quality data.
- The ICI camera provided the best results for plant phenotyping, as its discerning ability was shown to be higher than those of the FLIR and thermoMap. Although respectable results were achieved by the FLIR, the ICI provided a more thorough and accurate result.
- Higher heritability indicates that a greater portion of plant trait variations is the result of genetic differences. The heritability of plot mean temperatures was highest when calculated based on the ICI camera, followed by FLIR and then thermoMap, with values of 0.756, 0.744, and 0.729, respectively. All three cameras demonstrated that over 72% of variability in plot mean temperatures was accounted for by genetic differences.
- The best overall thermal camera for precision agriculture and phenotyping based on this study was the ICI, as it performed well, with appealing spatial data, a close performance in image quality, with the highest value being exhibited for heritability. This is consistent with the relative camera specifications that were claimed by manufacturers.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Sagan, V.; Maimaitiyiming, M.; Fishman, J. Effects of Ambient Ozone on Soybean Biophysical Variables and Mineral Nutrient Accumulation. Remote Sens. 2018, 10, 562. [Google Scholar] [CrossRef]
- Seguin, B.; Lagouarde, J.P.; Savane, M. The Assessment of Regional Crop Water Conditions from Meteorological Satellite Thermal Infrared Data. Remote Sens. Environ. 1991, 35, 141–148. [Google Scholar] [CrossRef]
- Bastiaanssen, W.G.M. SEBAL-based sensible and latent heat fluxes in the irrigated Gediz Basin, Turkey. J. Hydrol. 2000, 229, 87–100. [Google Scholar] [CrossRef]
- Norman, J.M.; Anderson, M.C.; Kustas, W.P.; French, A.N.; Mecikalski, J.; Torn, R.; Diak, G.R.; Schmugge, T.J.; Tanner, B.C.W. Remote sensing of surface energy fluxes at 10(1)-m pixel resolutions. Water Resour. Res. 2003, 39, 1775. [Google Scholar] [CrossRef]
- Xu, C.Y.; Qu, J.J.; Hao, X.J.; Cosh, M.H.; Prueger, J.H.; Zhu, Z.L.; Gutenberg, L. Downscaling of Surface Soil Moisture Retrieval by Combining MODIS/Landsat and In Situ Measurements. Remote Sens. 2018, 10, 210. [Google Scholar] [CrossRef]
- Wang, J.; Ling, Z.W.; Wang, Y.; Zeng, H. Improving spatial representation of soil moisture by integration of microwave observations and the temperature-vegetation-drought index derived from MODIS products. ISPRS J. Photogramm. 2016, 113, 144–154. [Google Scholar] [CrossRef]
- Leng, P.; Song, X.N.; Li, Z.L.; Wang, Y.W.; Wang, R.X. Toward the Estimation of Surface Soil Moisture Content Using Geostationary Satellite Data over Sparsely Vegetated Area. Remote Sens. 2015, 7, 4112–4138. [Google Scholar] [CrossRef] [Green Version]
- Sepulcre-Canto, G.; Zarco-Tejada, P.J.; Sobrino, J.A.; Berni, J.A.J.; Jimenez-Munoz, J.C.; Gastellu-Etchegorry, J.P. Discriminating irrigated and rainfed olive orchards with thermal ASTER imagery and DART 3D simulation. Agric. For. Meteorol. 2009, 149, 962–975. [Google Scholar] [CrossRef]
- Veysi, S.; Naseri, A.; Hamzeh, S.; Bartholomeus, H. A satellite based crop water stress index for irrigation scheduling in sugarcane fields. Agric. Water Manag. 2017, 189, 70–86. [Google Scholar] [CrossRef]
- Leroux, L.; Baron, C.; Zoungrana, B.; Traore, S.B.; Lo Seen, D.; Begue, A. Crop Monitoring Using Vegetation and Thermal Indices for Yield Estimates: Case Study of a Rainfed Cereal in Semi-Arid West Africa. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 347–362. [Google Scholar] [CrossRef]
- Anderson, M.C.; Hain, C.; Wardlow, B.; Pimstein, A.; Mecikalski, J.R.; Kustas, W.P. Evaluation of Drought Indices Based on Thermal Remote Sensing of Evapotranspiration over the Continental United States. J. Clim. 2011, 24, 2025–2044. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.C.; Allen, R.G.; Morse, A.; Kustas, W.P. Use of Landsat thermal imagery in monitoring evapotranspiration and managing water resources. Remote Sens. Environ. 2012, 122, 50–65. [Google Scholar] [CrossRef]
- Kogan, F.N. Application of Vegetation Index and Brightness Temperature for Drought Detection. Adv. Space Res. 1995, 15, 91–100. [Google Scholar] [CrossRef]
- Song, Y.; Fang, S.B.; Yang, Z.Q.; Shen, S.H. Drought indices based on MODIS data compared over a maize-growing season in Songliao Plain, China. J. Appl. Remote Sens. 2018, 12, 046003. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Vergara, O.; Araus, J.; Tarekegne, A.; Magorokosho, C.; Zarco-Tejada, P.; Hornero, A.; Albà, A.H.; Das, B.; Craufurd, P. Unmanned aerial platform-based multi-spectral imaging for field phenotyping of maize. Plant Methods 2015, 11, 35. [Google Scholar] [CrossRef]
- Wang, K.; Franklin, S.E.; Guo, X.; Cattet, M. Remote sensing of ecology, biodiversity and conservation: A review from the perspective of remote sensing specialists. Sensors 2010, 10, 9647–9667. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Walter, A.; Liebisch, F.; Hund, A. Plant phenotyping: From bean weighing to image analysis. Plant Methods 2015, 11, 14. [Google Scholar] [CrossRef]
- Sadras, V.O.; Rebetzke, G.J.; Edmeades, G.O. The phenotype and the components of phenotypic variance of crop traits. Field Crop. Res. 2013, 154, 255–259. [Google Scholar] [CrossRef]
- Lake, L.; Li, Y.L.; Casal, J.J.; Sadras, V.O. Negative association between chickpea response to competition and crop yield: Phenotypic and genetic analysis. Field Crop. Res. 2016, 196, 409–417. [Google Scholar] [CrossRef]
- Madec, S.; Baret, F.; de Solan, B.; Thomas, S.; Dutartre, D.; Jezequel, S.; Hemmerle, M.; Colombeau, G.; Comar, A. High-Throughput Phenotyping of Plant Height: Comparing Unmanned Aerial Vehicles and Ground LiDAR Estimates. Front. Plant Sci. 2017, 8, 2002. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.J.; Liu, J.G.; Zhao, C.J.; Li, Z.H.; Huang, Y.B.; Yu, H.Y.; Xu, B.; Yang, X.D.; Zhu, D.M.; Zhang, X.Y.; et al. Unmanned Aerial Vehicle Remote Sensing for Field-Based Crop Phenotyping: Current Status and Perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Ghulam, A.; Sidike, P.; Hartling, S.; Maimaitiyiming, M.; Peterson, K.; Shavers, E.; Fishman, J.; Peterson, J.; Kadam, S.; et al. Unmanned Aerial System (UAS)-based phenotyping of soybean using multi-sensor data fusion and extreme learning machine. ISPRS J. Photogramm. 2017, 134, 43–58. [Google Scholar] [CrossRef]
- Jin, X.L.; Liu, S.Y.; Baret, F.; Hemerle, M.; Comar, A. Estimates of plant density of wheat crops at emergence from very low altitude UAV imagery. Remote Sens. Environ. 2017, 198, 105–114. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Thomasson, J.A.; Murray, S.C.; Pugh, N.A.; Rooney, W.L.; Shafian, S.; Rajan, N.; Rouze, G.; Morgan, C.L.S.; Neely, H.L.; et al. Unmanned Aerial Vehicles for High-Throughput Phenotyping and Agronomic Research. PLoS ONE 2016, 11, e0159781. [Google Scholar] [CrossRef]
- Espinoza, C.Z.; Khot, L.R.; Sankaran, S.; Jacoby, P.W. High Resolution Multispectral and Thermal Remote Sensing-Based Water Stress Assessment in Subsurface Irrigated Grapevines. Remote Sens. 2017, 9, 961. [Google Scholar] [CrossRef]
- Bellvert, J.; Zarco-Tejada, P.J.; Marsal, J.; Girona, J.; Gonzalez-Dugo, V.; Fereres, E. Vineyard irrigation scheduling based on airborne thermal imagery and water potential thresholds. Aust. J. Grape Wine R. 2016, 22, 307–315. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Goldhamer, D.; Zarco-Tejada, P.J.; Fereres, E. Improving the precision of irrigation in a pistachio farm using an unmanned airborne thermal system. Irrig. Sci. 2015, 33, 43–52. [Google Scholar] [CrossRef]
- Park, S.; Ryu, D.; Fuentes, S.; Chung, H.; Hernyndez-Montes, E.; O’Connell, M. Adaptive Estimation of Crop Water Stress in Nectarine and Peach Orchards Using High-Resolution Imagery from an Unmanned Aerial Vehicle (UAV). Remote Sens. 2017, 9, 828. [Google Scholar] [CrossRef]
- Di Gennaro, S.F.; Matese, A.; Gioli, B.; Toscano, P.; Zaldei, A.; Palliotti, A.; Genesio, L. Multisensor approach to assess vineyard thermal dynamics combining high resolution unmanned aerial vehicle (UAV) remote sensing and wireless sensor network (WSN) proximal sensing. Sci. Hortic. Amst. 2017, 221, 83–87. [Google Scholar] [CrossRef]
- Berni, J.A.J.; Zarco-Tejada, P.J.; Sepulcre-Canto, G.; Fereres, E.; Villalobos, F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Sullivan, D.G.; Fulton, J.P.; Shaw, J.N.; Bland, G. Evaluating the sensitivity of an unmanned thermal infrared aerial system to detect water stress in a cotton canopy. Trans. ASABE 2007, 50, 1955–1962. [Google Scholar] [CrossRef]
- Han, M.; Zhang, H.H.; DeJonge, K.C.; Comas, L.H.; Trout, T.J. Estimating maize water stress by standard deviation of canopy temperature in thermal imagery. Agric. Water Manag. 2016, 177, 400–409. [Google Scholar] [CrossRef]
- Hoffmann, H.; Jensen, R.; Thomsen, A.; Nieto, H.; Rasmussen, J.; Friborg, T. Crop water stress maps for an entire growing season from visible and thermal UAV imagery. Biogeosciences 2016, 13, 6545–6563. [Google Scholar] [CrossRef] [Green Version]
- Ludovisi, R.; Tauro, F.; Salvati, R.; Khoury, S.; Mugnozza, G.S.; Harfouche, A. UAV-Based Thermal Imaging for High-Throughput Field Phenotyping of Black Poplar Response to Drought. Front. Plant Sci. 2017, 8, 1681. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Candon, D.; Virlet, N.; Labbe, S.; Jolivot, A.; Regnard, J.L. Field phenotyping of water stress at tree scale by UAV-sensed imagery: New insights for thermal acquisition and calibration. Precis Agric. 2016, 17, 786–800. [Google Scholar] [CrossRef]
- Tattaris, M.; Reynolds, M.P.; Chapman, S.C. A Direct Comparison of Remote Sensing Approaches for High-Throughput Phenotyping in Plant Breeding. Front. Plant Sci. 2016, 7, 1131. [Google Scholar] [CrossRef]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant-environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Villegas, V.; Houx, J.H.; Singh, S.K.; Fritschi, F.B. Ground-Based Digital Imaging as a Tool to Assess Soybean Growth and Yield. Crop Sci. 2014, 54, 1756–1768. [Google Scholar] [CrossRef]
- Burnette, M.; Willis, C.; Kooper, R.; Maloney, J.D.; Ward, R.; Shakoor, N.; Newcomb, M.; Rohde, G.S.; Fahlgren, N.; Sagan, S.; et al. TERRA-REF Data Processing Infrastructure. In Proceedings of the Practice and Experience on Advanced Research Computing, Pittsburgh, PA, USA, 22–26 July 2018; p. 7. [Google Scholar]
- Turner, D.; Lucieer, A.; Watson, C. An Automated Technique for Generating Georectified Mosaics from Ultra-High Resolution Unmanned Aerial Vehicle (UAV) Imagery, Based on Structure from Motion (SfM) Point Clouds. Remote Sens. 2012, 4, 1392–1410. [Google Scholar] [CrossRef] [Green Version]
- Albertz, J. Einführung in die Fernerkundung: Grundlagen der Interpretation von Luft-und Satellitenbildern; Wiss. Buchges.: Darmstadt, Germany, 2001. [Google Scholar]
- Gago, J.; Fernie, A.R.; Nikoloski, Z.; Tohge, T.; Martorell, S.; Escalona, J.M.; Ribas-Carbo, M.; Flexas, J.; Medrano, H. Integrative field scale phenotyping for investigating metabolic components of water stress within a vineyard. Plant Methods 2017, 13, 90. [Google Scholar] [CrossRef]
- Berni, J.A.; Zarco-Tejada, P.J.; Suárez Barranco, M.D.; Fereres Castiel, E. Thermal and Narrow-Band Multispectral Remote Sensing for Vegetation Monitoring from an Unmanned Aerial Vehicle; Institute of Electrical and Electronics Engineers: Hoes Lane Piscataway, NJ, USA, 2009. [Google Scholar]
- Agudo, P.; Pajas, J.; Pérez-Cabello, F.; Redón, J.; Lebrón, B. The Potential of Drones and Sensors to Enhance Detection of Archaeological Cropmarks: A Comparative Study Between Multi-Spectral and Thermal Imagery. Drones 2018, 2, 29. [Google Scholar] [CrossRef]
- Raeva, P.L.; Šedina, J.; Dlesk, A. Monitoring of crop fields using multispectral and thermal imagery from UAV. Eur. J. Remote Sens. 2018, 1–10. [Google Scholar] [CrossRef]
- Harvey, M.C.; Rowland, J.V.; Luketina, K.M. Drone with thermal infrared camera provides high resolution georeferenced imagery of the Waikite geothermal area, New Zealand. J. Volcanol. Geotherm. Res. 2016, 325, 61–69. [Google Scholar] [CrossRef]
- Aubrecht, D.M.; Helliker, B.R.; Goulden, M.L.; Roberts, D.A.; Still, C.J.; Richardson, A.D. Continuous, long-term, high-frequency thermal imaging of vegetation: Uncertainties and recommended best practices. Agric. For. Meteorol. 2016, 228, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Torres-Rua, A. Vicarious calibration of sUAS microbolometer temperature imagery for estimation of radiometric land surface temperature. Sensors 2017, 17, 1499. [Google Scholar] [CrossRef]
- Sugiura, R.; Noguchi, N.; Ishii, K. Correction of low-altitude thermal images applied to estimating soil water status. Biosyst. Eng. 2007, 96, 301–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Meng, L.; Li, M.; Ding, L.; Ma, J. A Method for Deriving Plant Temperature from UAV TIR Image. In Proceedings of the 2018 7th International Conference on Agro-geoinformatics (Agro-geoinformatics), Hangzhou, China, 6–9 August 2018; pp. 1–5. [Google Scholar]
- Bergkamp, B.; Impa, S.; Asebedo, A.; Fritz, A.; Jagadish, S.K. Prominent winter wheat varieties response to post-flowering heat stress under controlled chambers and field based heat tents. Field Crop. Res. 2018, 222, 143–152. [Google Scholar] [CrossRef]
- Kraaijenbrink, P.D.A.; Shea, J.M.; Litt, M.; Steiner, J.F.; Treichler, D.; Koch, I.; Immerzeel, W.W. Mapping Surface Temperatures on a Debris-Covered Glacier With an Unmanned Aerial Vehicle. Front. Earth Sci. 2018, 6, 64. [Google Scholar] [CrossRef]
- Van der Sluijs, J.; Kokelj, S.V.; Fraser, R.H.; Tunnicliffe, J.; Lacelle, D. Permafrost Terrain Dynamics and Infrastructure Impacts Revealed by UAV Photogrammetry and Thermal Imaging. Remote Sens. 2018, 10, 1734. [Google Scholar] [CrossRef]
- Vollath, D. Automatic focusing by correlative methods. J. Microsc. 1987, 147, 279–288. [Google Scholar] [CrossRef]
- Crete, F.; Dolmiere, T.; Ladret, P.; Nicolas, M. The blur effect: Perception and estimation with a new no-reference perceptual blur metric. Proc. SPIE 2007, 64920I. [Google Scholar]
- Mittal, A.; Soundararajan, R.; Bovik, A.C. Making a” Completely Blind” Image Quality Analyzer. IEEE Signal Process. Lett. 2013, 20, 209–212. [Google Scholar] [CrossRef]
- Santos, A.; De Solorzano, C.O.; Vaquero, J.J.; Pena, J.M.; Malpica, N.; Del Pozo, F. Evaluation of autofocus functions in molecular cytogenetic analysis. J Microsc-Oxford 1997, 188, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Cullis, B.R.; Smith, A.B.; Coombes, N.E. On the design of early generation variety trials with correlated data. J. Agric. Biol. Environ. Stat. 2006, 11, 381–393. [Google Scholar] [CrossRef]
- Makanza, R.; Zaman-Allah, M.; Cairns, J.E.; Magorokosho, C.; Tarekegne, A.; Olsen, M.; Prasanna, B.M. High-Throughput Phenotyping of Canopy Cover and Senescence in Maize Field Trials Using Aerial Digital Canopy Imaging. Remote Sens. 2018, 10, 330. [Google Scholar] [CrossRef]
- Sheng, H.; Chao, H.; Coopmans, C.; Han, J.; McKee, M.; Chen, Y. Low-cost UAV-based thermal infrared remote sensing: Platform, calibration and applications. In Proceedings of the 2010 IEEE/ASME International Conference on Mechatronic and Embedded Systems and Applications, Qingdao, China, 15–17 July 2010; pp. 38–43. [Google Scholar]
- Romano, G.; Zia, S.; Spreer, W.; Sanchez, C.; Cairns, J.; Araus, J.L.; Müller, J. Use of thermography for high throughput phenotyping of tropical maize adaptation in water stress. Comput. Electron. Agric. 2011, 79, 67–74. [Google Scholar] [CrossRef]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Hansen, P.; Schjoerring, J. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Araus, J.; Casadesus, J.; Royo, C. Relationship between growth traits and spectral vegetation indices in durum wheat. Crop Sci. 2002, 42, 1547–1555. [Google Scholar] [CrossRef]
- Babar, M.; Reynolds, M.; Van Ginkel, M.; Klatt, A.; Raun, W.; Stone, M. Spectral reflectance to estimate genetic variation for in-season biomass, leaf chlorophyll, and canopy temperature in wheat. Crop Sci. 2006, 46, 1046–1057. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef]
















| Parameters | ICI 8640 P | FLIR Vue Pro R 640 | thermoMap | FLIR TG167 |
|---|---|---|---|---|
| Spectral Range | 7–14 μm | 7.5–13.5 μm | 7.5–13.5 μm | 8–14 μm |
| Frame Rate | 30 Hz | 30 Hz | 7.5 Hz | 9 Hz |
| Accuracy | (+/−) 1 °C | (+/−) 5 °C | (+/−) 5 °C | (+/−) 1.5 °C |
| Data Format | jpeg, 16-bit TIFF, 32-bit TIFF | Radiometric jpeg, 14-bit TIFF | 16-bit TIFF | bitmap |
| Sensor Resolution | 640 × 512 | 640 × 512 | 640 × 512 | 80 × 60 |
| Radiometric Resolution | 14 bit | 14 bit | 14 bit | N/A |
| Power Consumption | <1 W | 2.1 W | 5W | N/A |
| Pixel Pitch | 17 um | 17 um | 17 um | N/A |
| Thermal Sensitivity (NETD) | 0.02 °C | 0.05 °C | 0.1 °C | 0.15 °C |
| Focus | Manual | focused to infinity | focused to infinity | focused to infinity |
| Focal length | 13 mm | 13 mm | 9 mm | N/A |
| f-stop | 1.0 | 1.25 | 1.4 | N/A |
| Weight (g) | 74.5 | 92.0–113.0 | 134.0 | 312 |
| Cameras | Biomass (g) | LAI | Grain Yield (g) | Height (cm) | SC (mmol/m2·s) |
|---|---|---|---|---|---|
| ICI (°C) | 0.15 | −0.23 * | −0.52 * | −0.26 ** | −0.68 ** |
| FLIR (°C) | −0.07 | −0.03 | −0.41 ** | −0.28 ** | −0.37 * |
| Parameters | LAI | Height (cm) | NBI | Chl | ||||
|---|---|---|---|---|---|---|---|---|
| Samples | 193 | 237 | 165 | 165 | ||||
| Cameras | With soil | No soil | With soil | No soil | With soil | No soil | With soil | No soil |
| ICI | −0.266 ** | −0.261 * | −0.597 ** | −0.520 ** | 0.212 ** | 0.290 ** | 0.165 * | 0.253 ** |
| FLIR | −0.196 ** | −0.142 * | −0.427 ** | −0.263 ** | −0.219 ** | −0.359 ** | −0.239 * | −0.373 ** |
| thermoMap | −0.130 | −0.132 | −0.440 ** | −0.465 ** | −0.081 | −0.060 | −0.110 | −0.078 |
| Test Site | Cameras | Evaluation Metric | |||
|---|---|---|---|---|---|
| NIQE | BM | VC | MVC | ||
| Forest Park, St. Louis, MO | ICI | 3.312 | 0.340 | 201.451 | 400.034 |
| FLIR | 4.031 | 0.355 | 163.206 | 337.699 | |
| Bradford, Columbia, MO | ICI | 3.911 | 0.316 | 161.337 | 325.846 |
| FLIR | 4.404 | 0.329 | 193.204 | 390.599 | |
| Maricopa, AZ | ICI | 4.449 | 0.346 | 272.267 | 552.679 |
| FLIR | 4.592 | 0.345 | 268.672 | 539.110 | |
| thermoMap | 5.678 | 0.3180 | 188.5250 | 382.812 | |
| Test Site | Cameras | Evaluation Metric | |||
|---|---|---|---|---|---|
| NIQE | BM | VC | MVC | ||
| Forest Park, St. Louis, MO | ICI | 3.059 | 0.359 | 838.689 | 1690.026 |
| FLIR | 3.555 | 0.293 | 188.537 | 362.207 | |
| Bradford, Columbia, MO | ICI | 4.100 | 0.330 | 688.574 | 1408.620 |
| FLIR | 4.082 | 0.321 | 580.750 | 1,156.152 | |
| Maricopa, AZ | ICI | 4.175 | 0.401 | 338.970 | 695.624 |
| FLIR | 4.634 | 0.366 | 313.269 | 638.899 | |
| thermoMap | 8.481 | 0.489 | 102.327 | 242.439 | |
| VIs | NDVI | GNDVI | NDRE | |||
|---|---|---|---|---|---|---|
| Cameras | With soil | No soil | With soil | No soil | With soil | No soil |
| ICI | −0.857 ** | −0.720 ** | −0.846 ** | −0.631 ** | −0.803 ** | −0.626 ** |
| FLIR | −0.632 ** | −0.371 ** | −0.642 ** | −0.341 ** | −0.615 ** | −0.302 ** |
| thermoMap | −0.763 ** | −0.749 ** | −0.775 ** | −0.631 ** | −0.637 ** | −0.468 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagan, V.; Maimaitijiang, M.; Sidike, P.; Eblimit, K.; Peterson, K.T.; Hartling, S.; Esposito, F.; Khanal, K.; Newcomb, M.; Pauli, D.; et al. UAV-Based High Resolution Thermal Imaging for Vegetation Monitoring, and Plant Phenotyping Using ICI 8640 P, FLIR Vue Pro R 640, and thermoMap Cameras. Remote Sens. 2019, 11, 330. https://doi.org/10.3390/rs11030330
Sagan V, Maimaitijiang M, Sidike P, Eblimit K, Peterson KT, Hartling S, Esposito F, Khanal K, Newcomb M, Pauli D, et al. UAV-Based High Resolution Thermal Imaging for Vegetation Monitoring, and Plant Phenotyping Using ICI 8640 P, FLIR Vue Pro R 640, and thermoMap Cameras. Remote Sensing. 2019; 11(3):330. https://doi.org/10.3390/rs11030330
Chicago/Turabian StyleSagan, Vasit, Maitiniyazi Maimaitijiang, Paheding Sidike, Kevin Eblimit, Kyle T. Peterson, Sean Hartling, Flavio Esposito, Kapil Khanal, Maria Newcomb, Duke Pauli, and et al. 2019. "UAV-Based High Resolution Thermal Imaging for Vegetation Monitoring, and Plant Phenotyping Using ICI 8640 P, FLIR Vue Pro R 640, and thermoMap Cameras" Remote Sensing 11, no. 3: 330. https://doi.org/10.3390/rs11030330
APA StyleSagan, V., Maimaitijiang, M., Sidike, P., Eblimit, K., Peterson, K. T., Hartling, S., Esposito, F., Khanal, K., Newcomb, M., Pauli, D., Ward, R., Fritschi, F., Shakoor, N., & Mockler, T. (2019). UAV-Based High Resolution Thermal Imaging for Vegetation Monitoring, and Plant Phenotyping Using ICI 8640 P, FLIR Vue Pro R 640, and thermoMap Cameras. Remote Sensing, 11(3), 330. https://doi.org/10.3390/rs11030330