Determination of Phycocyanin from Space—A Bibliometric Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Previous Reviews on Remote Sensing of Phycocyanin
2.2. Data Acquisition
2.3. Methodology
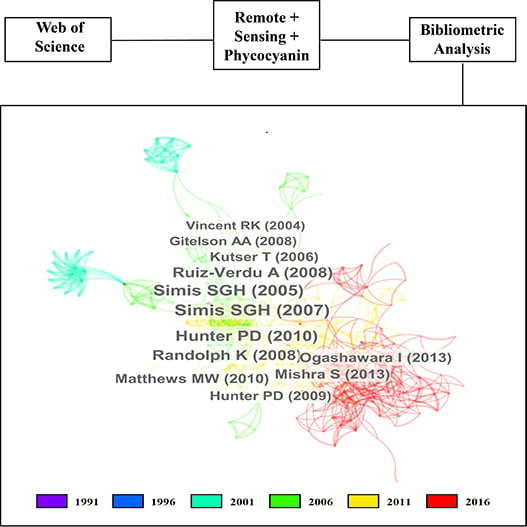
3. Results
3.1. 30 Years of Remote Sensing of Phycocianin
3.2. Decade-by-Decade Analysis
3.2.1. Period I (1991–2000)
3.2.2. Period II (2001–2010)
3.2.3. Period III (2011–2020)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Reference 1 | Journal | Citations | Year |
|---|---|---|---|
| Simis et al. (2007) | Remote Sensing of Environment | 38 | 2007 |
| Simis et al. | Limnology and Oceanography | 35 | 2005 |
| Hunter et al. | Remote Sensing of Environment | 31 | 2010 |
| Ruiz-Verdú et al. | Remote Sensing of Environment | 29 | 2008 |
| Randolph et al. | Remote Sensing of Environment | 29 | 2008 |
| Ogashawara et al. | Remote Sensing | 20 | 2013 |
| Matthews et al. (2010) | Remote Sensing of Environment | 19 | 2010 |
| Mishra et al. | Remote Sensing of Environment | 19 | 2013 |
| Hunter et al. | Environmental Science and Technology | 18 | 2009 |
| Kutser et al. (2006) | Estuarine, Coastal and Shelf Science | 18 | 2006 |
Appendix B
| Cluster ID | Top Label (LSI – LSI, p-value) | Top Label (LLR) |
|---|---|---|
| 0 | handheld spectroradiometer (29.03, 10−4); bloom management purposes (29.03, 10−4); turbid lake (24.84, 10−4); hybrid eof algorithm (24.84, 10−4); nonbloom condition (24.84, 10−4); modis cyanobacteria phycocyanin data (24.84, 10−4); dense coincident surface observation (20.67, 10−4); cyanobacterial total biovolume (20.67, 10−4); satellite reflectance algorithm (20.67, 10−4); temperate reservoir (20.67, 10−4); lake water quality (16.51, 10−4); | cyanobacteria; challenges; mapping cyanotoxin patterns; deep reservoir; drinking-water source; turbid lake; western basin; cyanobacterial total biovolume; evaluation; handheld spectroradiometer | lake erie; western basin; phytoplankton pigment absorption properties; regional example; cyanobacteria bloom waters; deep reservoir; drinking-water source; turbid lake; cyanobacterial total biovolume; evaluation |
| 1 | using genetic algorithm-partial leasts square (12.45, 0.001); potable water source (12.45, 0.001); hyperspectral retrieval (12.45, 0.001); microcystis aeruginosa (8.29, 0.005); eutrophic shallow lake (8.29, 0.005); case study (8.29, 0.005); spatial dynamics (8.29, 0.005); vertical migration (8.29, 0.005) | phycocyanin; modeling; hyperspectral retrieval; potable water sources; using genetic algorithm-partial least squares; suspended particulate matter; phytoplankton colour groups; spectral resolution; effect; sensor | suspended particulate matter; spectral discrimination; phytoplankton colour groups; spectral resolution; effect; sensor; using genetic algorithm-partial least squares; chlorophyll-a; modeling; current review |
| 2 | quasi-analytical algorithm (25.56, 10−4); retrieving absorption coefficient (18.06, 10−4); hyperspectral remote sensing reflectance (18.06, 10−4); multiple phytoplankton pigment (18.06, 10−4); organic matter (14.45, 0.001); cyanobacteria bloom water (12.94, 0.001); tropical eutrophic water (10.92, 0.001); mapping cyanobacterial bloom (10.05, 0.005); lake erie (8.13, 0.005) | quasi-analytical algorithm; parametrization; calibration; tropical eutrophic waters; cyanobacteria; mapping cyanotoxin patterns; challenges; semi-analytical algorithm; cyanobacteria biovolume; phycocyanin | cyanobacteria; challenges; mapping cyanotoxin patterns; using vertical cumulative pigment concentration; deep reservoir; phycocyanin; cyanobacteria biovolume; semi-analytical algorithm; remote estimation; meris sensor |
| 3 | cyanobacterial pigment (17.52, 10−4); theoretical basis (13.35, 0.001); cyanobacterial phycocyanin pigment concentration (13.35, 0.001); practical consideration (13.35, 0.001); eutrophic lake (13.29, 0.001); cell population (9.3, 0.005); using ocm satellite data (9.03, 0.005); freshwater lake (9.03, 0.005) | cyanobacterial pigments; freshwater lake; estimation; using ocm satellite data; cell populations; lake erie; evaluating multiple colour-producing agents; case ii waters; chlorophyll-a; mineral matter | mineral matter; cdom; absorption coefficients; central indiana reservoirs; chlorophyll; determination; cyanobacterial pigments; freshwater lake; estimation; using ocm satellite data |
| 4 | monitoring cyanobacterial bloom (16.35, 10−4); recognising cyanobacterial bloom (8.12, 0.005); modelling study (8.12, 0.005); optical signature (8.12, 0.005) | fluorescence characteristics; salinity gradient; phytoplankton; spectral absorption; different size fractions; baltic sea; recognising cyanobacterial blooms; modelling study; monitoring cyanobacterial blooms; satellite | recognising cyanobacterial blooms; modelling study; optical signature; fluorescence characteristics; salinity gradient; phytoplankton; spectral absorption; different size fractions; baltic sea; monitoring cyanobacterial blooms |
| 5 | china; lake; seasonal-spatial variation; shallow lake; phytoplankton absorption; multidecadal time series; south; satellite-detected accumulations; portugal; pigment c-phycocyanin | evaluation; east china; several lakes; spring bloom formation; remote sensing algorithms; cyanobacterial pigment retrievals; multidecadal time series; south; satellite-detected accumulations; portugal | |
| 6 | cyanobacterial biomass (32.04, 10−4); phycocyanin detection (27.56, 10−4); landsat tm data (27.56, 10−4); phytoplankton pigment composition (23.17, 10−4); cyanobacterial pigment phycocyanin (18.89, 10−4); spectral absorption (14.98, 0.001); different size fraction (14.98, 0.001); fluorescence characteristics (14.98, 0.001); salinity gradient (14.98, 0.001); phytoplankton absorption spectra (14.83, 0.001); inverse modeling approach (14.83, 0.001); phycocyanin pigment (14.74, 0.001); lake erie (10.85, 0.001); lake taihu (10.68, 0.005); baltic sea (9.41, 0.005); mapping cyanobacterial bloom (7.96, 0.005) | cyanobacterial biomass; algorithms; evaluation; phytoplankton absorption spectra; inverse modeling approach; estimating phytoplankton pigment concentrations; fluorescence characteristics; phytoplankton; spectral absorption; baltic sea | fluorescence characteristics; phytoplankton; spectral absorption; baltic sea; salinity gradient; different size fractions; algorithms; cyanobacterial biomass; cyanobacterial pigment phycocyanin; lake erie |
| 7 | cyanobacterial bloom (20.55, 10−4); user need (19.04, 10−4); future development (19.04, 10−4); multidisciplinary remote sensing ocean color sensor (19.04, 10−4); aquatic ecosystem (16.14, 10−4); hyperspectral global mapping satellite mission (16.14, 10−4); measuring freshwater (16.14, 10−4); floating algae index (13.35, 0.001); monitoring level (13.35, 0.001); visual cyanobacteria index (13.35, 0.001); multiscale mapping assessment (10.68, 0.005); cyanobacterial harmful algal bloom (10.68, 0.005); mapping cyanobacterial bloom (10.62, 0.005); lake erie (8.59, 0.005) | waters; semi-analytical algorithm; phycocyanin; remote estimation; deep reservoir; algorithms; modeling; comparative review; new scheme; user needs | new scheme; complex turbid; hyperspectral reflectance; implications; test; inversion algorithms; reconstruction; cyanobacterial harmful algal blooms; multiscale mapping assessment; lake champlain |
| 9 | great lake (15.82, 10−4); using modis (15.82, 10−4); mapping cyanobacterial bloom (14.44, 0.001); drinking-water source (12.43, 0.001); modis observation (12.43, 0.001); cyanobacterial risk (12.43, 0.001); long-term safety evaluation (12.43, 0.001); case ii water (10.33, 0.005); evaluating multiple colour-producing agent (10.33, 0.005); meris satellite data (8.23, 0.005); using quickbird (8.23, 0.005); missisquoi bay (8.23, 0.005) | mapping cyanobacterial blooms; meris satellite data; using quickbird; current review; near-coastal transitional waters; empirical procedures; lake erie; evaluating multiple colour-producing agents; case ii waters; using modis | drinking-water source; cyanobacterial risks; implications; modis observations; long-term safety evaluation; eutrophic lake; complex waters; cyanobacteria abundance; ocean colour estimation; inherent optical properties |
| 11 | active pigment (22.35, 10−4); turbid productive water (22.35, 10−4); mesotrophic reservoir (13.61, 0.001); predicting phycocyanin concentration (9.45, 0.005); novel algorithm (9.45, 0.005); | cyanobacteria; predicting phycocyanin concentrations; novel algorithm; proximal hyperspectral remote sensing approach; mesotrophic reservoir; phycocyanin; turbid productive water; chlorophyll; chlorophyll-a | phycocyanin; turbid productive water; chlorophyll; proximal hyperspectral remote sensing approach; predicting phycocyanin concentrations; chlorophyll-a; mesotrophic reservoir; cyanobacteria; novel algorithm |
References
- Bartram, J.; Carmichael, W.W.; Chorus, I.; Jones, G.; Skulberg, O.M. Introduction. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 1st ed.; Chorus, I., Bartram, J., Eds.; UNESCO/WHO/UNEP: London, UK, 1999; Chapter 1; pp. 12–24. [Google Scholar]
- Codd, G.A.; Lindsay, J.; Young, F.M.; Morrison, L.F.; Metcalf, J.S. Harmful Cyanobacteria. In Harmful Cyanobacteria, 1st ed.; Huisman, J., Matthijs, H.C.P., Visser, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2005; Chapter 1; pp. 1–23. [Google Scholar]
- Sivonen, K.; Jones, G. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 1st ed.; Chorus, I., Bartram, J., Eds.; UNESCO/WHO/UNEP: London, UK, 1999; Chapter 3; pp. 55–124. [Google Scholar]
- Carmichael, W.W. Health effects of toxin producing cyanobacteria: The cyanoHABs. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Li, L.; Sengpiel, R.E.; Pascual, D.L.; Tedesco, L.D.; Wilson, J.S.; Soyeux, E. Using hyperspectral remote sensing to estimate chlorophyll-a and phycocyanin in a mesotrophic reservoir. Int. J. Remote Sens. 2010, 31, 4147–4162. [Google Scholar] [CrossRef]
- Le, C.; Li, Y.; Zha, Y.; Wang, Q.; Zhang, H.; Yin, B. Remote sensing of phycocyanin pigment in highly turbid inland waters in Lake Taihu, China. Int. J. Remote Sens. 2011, 32, 8253–8269. [Google Scholar] [CrossRef]
- Matthews, M.W.; Odermatt, D. Improved algorithm for routine monitoring of cyanobacteria and eutrophication in inland and near-coastal waters. Remote Sens. Environ. 2015, 156, 374–382. [Google Scholar] [CrossRef]
- Reynolds, C.S. Cyanobacterial Water-Blooms. In Advances in Botanical Research; Callow, J.A., Ed.; Academic Press Inc.: London, UK, 1987; Volume 13, pp. 67–143. [Google Scholar] [CrossRef]
- Hyenstrand, P.; Blomqvist, P.; Pettersson, A. Factors determining cyanobacterial success in aquatic systems: A literature review. Arch. Hydrobiol. 1998, 51, 41–62. [Google Scholar]
- Paerl, H.W. Marine Plankton. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 127–153. [Google Scholar]
- Duan, H.; Ma, R.; Xu, J.; Zhang, Y.; Zhang, B. Comparison of different semi-empirical algorithms to estimate chlorophyll-a concentration in inland lake water. Environ. Monit. Assess. 2010, 170, 231–244. [Google Scholar] [CrossRef]
- Gons, H.J. Optical Teledetection of Chlorophyll a in Turbid Inland Waters. Environ. Sci. Technol. 1999, 33, 1127–1132. [Google Scholar] [CrossRef]
- Hadijimitsis, D.G.; Clayton, C. Assessment of temporal variations of water quality in inland water bodies using atmospheric corrected satellite remotely sensed image data. Environ. Monit. Assess. 2009, 159, 281–292. [Google Scholar] [CrossRef]
- Reinart, A.; Kutser, T. Comparison of different satellite sensors in detecting cyanobacterial bloom events in the Baltic Sea. Remote Sens. Environ. 2006, 102, 74–85. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Gilvear, D.J.; Willby, N.J. Using remote sensing to aid the assessment of human health risks from blooms of potentially toxic cyanobacteria. Environ. Sci. Technol. 2009, 43, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Vincent, R.K.; Qin, X.; McKay, R.M.L.; Miner, J.; CzajkowskI, K.; Savino, J.; Bridgeman, T. Phycocyanin detection from LANDSAT TM data for mapping cyanobacterial blooms in Lake Erie. Remote Sens. Environ. 2004, 89, 381–392. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R.; Schluchter, W.M. A Novel algorithm for predicting phycocyanin concentrations in cyanobacteria: A proximal hyperspectral remote sensing approach. Remote Sens. 2009, 1, 758–775. [Google Scholar] [CrossRef] [Green Version]
- Ogashawara, I.; Mishra, D.; Mishra, S.; Curtarelli, M.; Stech, J. A Performance Review of Reflectance Based Algorithms for Predicting Phycocyanin Concentrations in Inland Waters. Remote Sens. 2013, 5, 4774–4798. [Google Scholar] [CrossRef] [Green Version]
- Stumpf, R.; Dupuy, D. Experimental Lake Erie Harmful Algal Bloom Bulletin—Bulletin 28/2016; NOAA: Washington, DC, USA, 2016.
- Cyanobacteria Assessment Network (CyAN). Available online: https://www.epa.gov/water-research/cyanobacteria-assessment-network-cyan#satellitealgorithms (accessed on 24 November 2017).
- IIWQ Water Quality Portal. Available online: http://www.worldwaterquality.org/ (accessed on 16 January 2020).
- Ruiz-Verdú, A.; Simis, S.G.H.; de Hoyos, C.; Gons, H.J.; Peña-Martínez, R. An evaluation of algorithms for the remote sensing of cyanobacterial biomass. Remote Sens. Environ. 2008, 112, 3996–4008. [Google Scholar] [CrossRef]
- Beck, R.; Xu, M.; Zhan, S.; Liu, H.; Johansen, R.; Tong, S.; Yang, B.; Shu, S.; Wu, Q.; Wang, S.; et al. Comparison of Satellite Reflectance Algorithms for Estimating Phycocyanin Values and Cyanobacterial Total Biovolume in a Temperate Reservoir Using Coincident Hyperspectral Aircraft Imagery and Dense Coincident Surface Observations. Remote Sens. 2017, 9, 538. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Bao, Z.; Shao, J. Phycocyanin concentration retrieval in inland waters: A comparative review of the remote sensing techniques and algorithms. J. Great Lakes Res. 2018, 44, 748–755. [Google Scholar] [CrossRef]
- Riddick, C.A.L.; Hunter, P.D.; Domínguez-Gómez, J.A.; Martinez-Vicente, V.; Présing, M.; Horváth, H.; Kovács, A.W.; Vörös, L.; Zsigmond, E.; Tyler, A.N. Optimal Cyanobacterial Pigment Retrieval from Ocean Colour Sensors in a Highly Turbid, Optically Complex Lake. Remote Sens. 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Zhang, Y.; Qin, B.; Zhou, B. Remote sensing of cyanobacterial blooms in inland waters: Present knowledge and future challenges. Sci. Bull. 2019, 64, 1540–1556. [Google Scholar] [CrossRef] [Green Version]
- Schalles, J.F.; Yacobi, Y.Z. Remote detection and seasonal patterns of phycocyanin, carotenoid and chlorophyll pigments in eutrophic waters. Ergeb. Limnol. 2000, 55, 153–168. [Google Scholar]
- Mishra, S.; Mishra, D.R.; Lee, Z.; Tucker, C.S. Quantifying cyanobacterial phycocyanin concentration in turbid productive waters: A quasi-analytical approach. Remote Sens. Environ. 2013, 133, 141–151. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Song, K. Remote sensing of freshwater cyanobacteria: An extended IOP Inversion Model of Inland Waters (IIMIW) for partitioning absorption coefficient and estimating phycocyanin. Remote Sens. Environ. 2015, 157, 9–23. [Google Scholar] [CrossRef]
- Dekker, A.G. Detection of Optical Water Quality Parameters for Eutrophic Waters by High Resolution Remote Sensing. Ph.D. Thesis, Proefschrift Vrije Universiteit (Free University), Amsterdam, The Netherlands, 1993. [Google Scholar]
- Hunter, P.D.; Tyler, A.N.; Carvalho, L.; Codd, G.A.; Maberly, S.C. Hyperspectral remote sensing of cyanobacterial pigments as indicators for cell populations and toxins in eutrophic lakes. Remote Sens. Environ. 2010, 114, 2705–2718. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S. Remote Sensing of Cyanobacteria in Turbid Productive Waters. Ph.D. Thesis, Mississippi State University, Starkville, MS, USA, 2012. [Google Scholar]
- Mishra, S.; Mishra, D.R. A novel remote sensing algorithm to quantify phycocyanin in cyanobacterial algal blooms. Environ. Res. Lett. 2014, 9, 114003. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Davis, T.W.; Wynne, T.T.; Graham, J.L.; Loftin, K.A.; Johengen, T.H.; Gossiaux, D.; Palladino, D.; Burtner, A. Challenges for mapping cyanotoxin patterns from remote sensing of cyanobacteria. Harmful Algae 2016, 54, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Warner, R.A.; Tester, P.A.; Dyble, J.; Fahnenstiel, G.L. Relating spectral shape to cyanobacterial blooms in the Laurentian Great Lakes. Int. J. Remote Sens. 2008, 29, 3665–3672. [Google Scholar] [CrossRef]
- Dash, P.; Walker, N.D.; Mishra, D.R.; Hu, C.; Pinckney, J.L.; D’Sa, E.J. Estimation of cyanobacterial pigments in a freshwater lake using OCM satellite data. Remote Sens. Environ. 2011, 115, 3409–3423. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Shi, K.; Li, Z.; Song, K. A semi-analytical algorithm for remote estimation of phycocyanin in inland waters. Sci. Total Environ. 2012, 435, 141–150. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Duan, H.; Cannizzaro, J.; Ma, R. A novel MERIS algorithm to derive cyanobacterial phycocyanin pigment concentrations in a eutrophic lake: Theoretical basis and practical considerations. Remote Sens. Environ. 2014, 154, 298–317. [Google Scholar] [CrossRef]
- Liu, G.; Simis, S.G.H.; Li, L.; Wang, Q.; Li, Y.; Song, K.; Lyu, H.; Zheng, Z.; Shi, K. A Four-Band Semi-Analytical Model for Estimating Phycocyanin in Inland Waters From Simulated MERIS and OLCI Data. IEEE Trans. Geosci. Remote Sens. 2018, 56, 1374–1385. [Google Scholar] [CrossRef]
- Hunter, P.; Tyler, A.; Presing, M.; Kovacs, A.; Preston, T. Spectral discrimination of phytoplankton colour groups: The effect of suspended particulate matter and sensor spectral resolution. Remote Sens. Environ. 2008, 112, 1527–1544. [Google Scholar] [CrossRef]
- Randolph, K.; Wilson, J.; Tedesco, L.; Li, L.; Pascual, D.L.; Soyeux, E. Hyperspectral remote sensing of cyanobacteria in turbid productive water using optically active pigments, chlorophyll a and phycocyanin. Remote Sens. Environ. 2008, 112, 4009–4019. [Google Scholar] [CrossRef]
- Duan, H.; Ma, R.; Hu, C. Evaluation of remote sensing algorithms for cyanobacterial pigment retrievals during spring bloom formation in several lakes of East China. Remote Sens. Environ. 2012, 126, 126–135. [Google Scholar] [CrossRef]
- Wheeler, S.M.; Morrissey, L.A.; Levine, S.N.; Livingston, G.P.; Vincent, W.F. Mapping cyanobacterial blooms in Lake Champlain’s Missisquoi Bay using QuickBird and MERIS satellite data. J. Great Lakes Res. 2012, 38, 68–75. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Wang, Q.; Gao, J.; Le, C.; Huang, C.; Gong, S. Hyperspectral Remote Sensing of the Pigment C-Phycocyanin in Turbid Inland Waters, Based on Optical Classification. IEEE Trans. Geosci. Remote Sens. 2013, 51, 3871–3884. [Google Scholar] [CrossRef]
- Wozniak, M.; Bradtke, K.M.; Darecki, M.; Krężel, A. Empirical model for phycocyanin concentration estimation as an indicator of cyanobacterial bloom in the optically complex coastal waters of the Baltic Sea. Remote Sens. 2016, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101, 5303–5310. [Google Scholar] [CrossRef] [Green Version]
- Chen, C. Citespace ii: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef] [Green Version]
- Small, H. Co-citation in the scientific literature: A new measure of the relationship between two documents. J. Am. Soc. Inf. Sci. 1973, 24, 265–269. [Google Scholar] [CrossRef]
- Ogashawara, I.; Alcântara, E.H.; Stech, J.L.; Tundisi, J.G. Cyanobacterial Detection in Guarapiranga Reservoir (São Paulo State, Brazil) From Landsat TM and ETM + Images. Rev. Ambiente. Água 2014, 9, 224–238. [Google Scholar] [CrossRef] [Green Version]
- Oyama, Y.; Matsushita, B.; Fukushima, T. Distinguishing surface cyanobacterial blooms and aquatic macrophytes using landsat/TM and ETM + shortwave infrared bands. Remote Sens. Environ. 2015, 157, 35–47. [Google Scholar] [CrossRef]
- Francis, G. Poisonous Australian Lake. Nature 1878, 18, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Chapra, S.C.; Boehlert, B.; Fant, C.; Bierman, V.J., Jr.; Henderson, J.; Mills, D.; Mas, D.M.L.; Rennels, L.; Jantarasami, L.; Martinich, J.; et al. Climate Change Impacts on Harmful Algal Blooms in U.S. Freshwaters: A Screening-Level Assessment. Environ. Sci. Technol. 2017, 51, 8933–8943. [Google Scholar] [CrossRef] [PubMed]
- Greb, S.; Dekker, A.; Binding, C. (Eds.) Earth Observations in Support of Global Water Quality Monitoring; IOCCG Report Series, No. 17; International Ocean Colour Coordinating Group: Dartmouth, NS, Canada, 2018. [Google Scholar]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Matisoff, G.; Kaltenberg, E.M.; Steely, R.L.; Hummel, S.K.; Seo, J.; Gibbons, K.J.; Bridgeman, T.B.; Seo, Y.; Behbahani, M.; James, W.F.; et al. Internal loading of phosphorus in western Lake Erie. J. Great Lakes Res. 2016, 42, 775–788. [Google Scholar] [CrossRef]
- Ashton, P.J. Seasonality in Southern Hemisphere freshwater phytoplankton assemblages. Hydrobiologia 1985, 125, 179–190. [Google Scholar] [CrossRef]
- Moses, W.J.; Sterckx, S.; Montes, L.; Keukelaere, L.D.; Knaeps, E. Atmospheric correction for inland waters. In Bio-Optical Modeling and Remote Sensing of Inland Waters, 1st ed.; Mishra, D.R., Ogashawara, I., Gitelson, A.A., Eds.; Elsevier: New York, NY, USA, 2017; Chapter 3; pp. 69–100. [Google Scholar]






| Study 1 | Study Sites | Reviewed Algorithms 2 |
|---|---|---|
| Ruiz-Verdu et al. | Spanish and Dutch Lakes and Reservoirs | DE93, SC00, SI05 |
| Ogashawara et al. | Funil Hydroelectric Reservoir (Brazil) and Catfish Ponds (USA) | DE93, SC00, SI05, MI09, HU10, MI12 |
| Beck et al. | Harsha Lake (USA) | SC00, SI05, HU10, MI12, MI14, ST16 |
| Yan et al. | - | DE93, SC00, SI05, WY08, MI09, HU10, DA11, LI12, MI13, QI14, MI14 |
| Riddick et al. | Global Lakes (LIMNADES dataset) | DE93, SC00, SI05, HU10, MI13, QI14, LI15, LI18 |
| Shi et al. | - | SC00, VI04, SI05, HU08, RA08, RU08, HU10, DA11, DU12, LI12, WH12, MI13, MI14, QI14, SU15, WO16 |
| Cluster ID | Size | Main Label (LSI) | Main Label (LLR) | Mean (Cited Year) |
|---|---|---|---|---|
| 4 | 21 | Fluorescence characteristics | Monitoring Cyanobacteria Bloom (p-value = 10−4) | 1999 |
| 6 | 17 | Cyanobacterial Biomass | Cyanobacterial Biomass (p-value = 10−4) | 2001 |
| 11 | 7 | Cyanobacteria | Active Pigment (p-value = 10−4) | 2004 |
| 1 | 47 | Phycocyanin | Using genetic algorithm-partial least square (p-value = 10−3) | 2006 |
| 5 | 20 | China | Atmospheric Correction (p-value = 10−4) | 2007 |
| 3 | 30 | Cyanobacterial pigments | Cyanobacterial pigments (p-value = 10−4) | 2008 |
| 9 | 12 | Mapping cyanobacteria bloom | Great Lake (p-value = 10−4) | 2009 |
| 7 | 16 | Waters | Cyanobacteria Bloom (p-value = 10−4) | 2012 |
| 0 | 55 | Cyanobacteria | Handheld spectroradiometer (p-value = 10−4) | 2013 |
| 2 | 36 | Quasi-analytical algorithms | Quasi-analytical algorithms (p-value = 10−4) | 2013 |
| Cluster ID | Size | Main Label (LSI) | Main Label (LLR) | Mean (Cited Year) |
|---|---|---|---|---|
| 0 | 20 | High resolution airborne remote sensing | Optical properties of dense algal cultures (p-value = 0.5) | 1986 |
| 1 | 12 | Optical properties of dense algal cultures | High resolution airborne remote sensing (p-value = 0.5) | 1992 |
| Cluster ID | Size | Main Label (LSI) | Main Label (LLR) | Mean (Cited Year) |
|---|---|---|---|---|
| 2 | 20 | Spectral absorption and fluorescence characteristics | Different size fraction (p-value = 0.5) | 1999 |
| 1 | 22 | Landsat TM data | Lake Erie (p-value = 0.005) | 2000 |
| 11 | 7 | Fluorescence characteristics | Monitoring cyanobacterial bloom (p-value = 0.05) | 2001 |
| 6 | 11 | China | Hyperspectral retrieval model (p-value = 0.05) | 2002 |
| 10 | 8 | Cyanobacterial pigments | Cell population (p-value = 0.1) | 2003 |
| 8 | 9 | Spatial dynamics of vertical migration | Eutrophic shallow lake (p-value = 0.05) | 2003 |
| 0 | 24 | Cyanobacteria biomass | Cyanobacteria biomass (p-value = 0.005) | 2004 |
| 5 | 11 | Phycocyanin | Turbid productive waters (p-value = 0.001) | 2004 |
| 3 | 16 | Cyanobacteria pigments | Toxic cyanobacteria (p-value = 0.05) | 2005 |
| 4 | 14 | Phytoplankton absorption | Great Lake (p-value = 0.05) | 2005 |
| Cluster ID | Size | Main Label (LSI) | Main Label (LLR) | Mean (Cited Year) |
|---|---|---|---|---|
| 8 | 18 | Mineral matter characteristics | Near-coastal transitional water (p-value = 0.001) | 2006 |
| 0 | 48 | Modeling | Phycocyanin pigment (p-value = 10−4) | 2007 |
| 5 | 22 | Case 2 | Optical characterization (p-value = 10−4) | 2008 |
| 11 | 7 | Phycocyanin | Satellite-detected accumulation (p-value = 10−4) | 2010 |
| 7 | 19 | Waters | Using Landsat measurement (p-value = 10−4) | 2010 |
| 4 | 29 | New scheme | Theoretical basis (p-value = 0.01) | 2010 |
| 10 | 14 | Semi-analytical algorithm | Modern robust approach (p-value = 10−4) | 2012 |
| 1 | 41 | Chlorophyll-a prediction algorithms | Tropical eutrophic water (p-value = 10−4) | 2012 |
| 9 | 15 | Cyanobacterial total biovolume | Eastern Iberian Peninsula (p-value = 10−4) | 2013 |
| 3 | 30 | Lake Erie | Risk factor (p-value = 10−4) | 2013 |
| 2 | 36 | Drinking water source | Turbid lake (p-value = 10−4) | 2013 |
| 6 | 20 | Cyanobacterial Blooms | Deep reservoir (p-value = 10−4) | 2015 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogashawara, I. Determination of Phycocyanin from Space—A Bibliometric Analysis. Remote Sens. 2020, 12, 567. https://doi.org/10.3390/rs12030567
Ogashawara I. Determination of Phycocyanin from Space—A Bibliometric Analysis. Remote Sensing. 2020; 12(3):567. https://doi.org/10.3390/rs12030567
Chicago/Turabian StyleOgashawara, Igor. 2020. "Determination of Phycocyanin from Space—A Bibliometric Analysis" Remote Sensing 12, no. 3: 567. https://doi.org/10.3390/rs12030567
APA StyleOgashawara, I. (2020). Determination of Phycocyanin from Space—A Bibliometric Analysis. Remote Sensing, 12(3), 567. https://doi.org/10.3390/rs12030567