Effects of Inter- and Intra-Specific Interactions on Moose Habitat Selection Limited by Temperature
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Multi-Scale Habitat Selection Modeling for Moose and Roe Deer
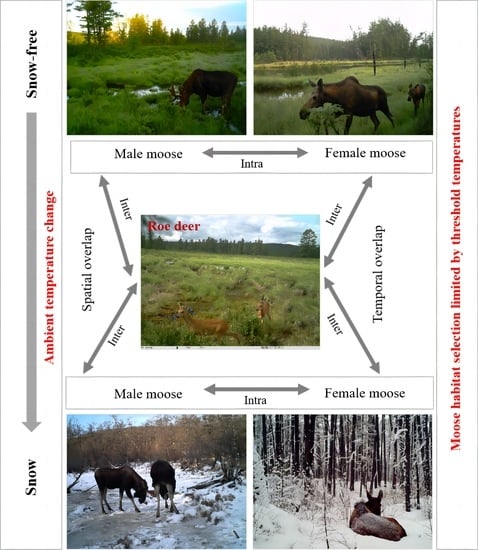
2.3. Spatial and Temporal Overlap for Moose and Roe Deer Occurrences
2.4. Temperature Effects on Female and Male Moose Population Distribution
3. Results
3.1. Univariate Optimal Scales of Moose and Roe Deer Occurrence
3.2. Habitat Selection of Female Moose, Male Moose, and Roe Deer
3.3. Spatial Overlap of Female Moose, Male Moose, and Roe Deer
3.4. Temporal Overlap of Female Moose, Male Moose, and Roe Deer
3.5. Relationships between Temperature and Moose Distribution
4. Discussion
4.1. Effect of Scale on Moose and Roe Deer Habitat Selection
4.2. Differences in Female and Male Moose Habitat Selection
4.3. Effect of Roe Deer Occurrences on Moose Distribution and Daily Activity
4.4. Effect of Temperature on Moose Distribution and Daily Activity Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Decesare, N.J.; Hebblewhite, M.; Bradley, M.; Hervieux, D.; Neufeld, L.; Musiani, M. Linking habitat selection and predation risk to spatial variation in survival. J. Anim. Ecol. 2013, 83, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.S.; Johnson, C.J.; Merrill, E.H.; Nielsen, S.E.; Solberg, E.J.; van Moorter, B. Review: Can habitat selection predict abundance? J. Anim. Ecol. 2016, 85, 11–20. [Google Scholar] [CrossRef]
- Morris, D.W. How can we apply theories of habitat selection to wildlife conservation and management? Wildl. Res. 2003, 30, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Niclas, J. Habitat selection: Implications for monitoring, management and conservation. Isr. J. Ecol. Evol. 2008, 54, 459–471. [Google Scholar]
- Timm, B.C.; McGarigal, K.; Cushman, S.A.; Ganey, J.L. Multi-scale mexican spotted owl (Strix occidentalis lucida) nest/roost habitat selection in arizona and a comparison with single-scale modeling results. Landsc. Ecol. 2016, 31, 1209–1225. [Google Scholar] [CrossRef]
- Wan, H.Y.; Mcgarigal, K.; Ganey, J.L.; Lauret, V.; Timm, B.C.; Cushman, S.A. Meta-replication reveals nonstationarity in multi-scale habitat selection of mexican spotted owl. Condor 2017, 119, 641–658. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Bothwell, H.M.; Hearn, A.J.; Cheyne, S.M.; Haidir, I.; Hunter, L.T.; Kaszta, Z.; Linkie, M.; Macdonald, E.A.; Ross, J.; et al. Multi-scale habitat selection modeling identifies threats and conservation opportunities for the Sunda clouded leopard (Neofelis diardi). Biol. Conserv. 2018, 227, 92–103. [Google Scholar] [CrossRef]
- McGarigal, K.; Wan, H.Y.; Zeller, K.A.; Timm, B.C.; Cushman, S.A. Multi-scale habitat selection modeling: A review and outlook. Landsc. Ecol. 2016, 31, 1161–1175. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Sinclair, A.R. Causes and consequences of migration by large herbivores. Trends Ecol. Evol. 1988, 3, 237–241. [Google Scholar] [CrossRef]
- Bao, H.; Fryxell, J.M.; Liu, H.; Dou, H.; Ma, Y.; Jiang, G. Effects of interspecific interaction-linked habitat factors on moose resource selection and environmental stress. Sci. Rep. 2017, 7, 41514. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Ma, J.; Stott, P.; Zhang, M. Multi-scale foraging habitat use and interactions by sympatric cervids in northeastern China. J. Wildl. Manag. 2010, 74, 678–689. [Google Scholar] [CrossRef]
- Long, R.A.; Rachlow, J.L.; Kie, J.G. Sex-specific responses of north american elk to habitat manipulation. J. Mammal. 2009, 90, 423–432. [Google Scholar] [CrossRef]
- Bowyer, R.T. Sexual segregation in ruminants: Definitions, hypotheses, and implications for conservation. J. Mammal. 2004, 85, 1039–1052. [Google Scholar] [CrossRef] [Green Version]
- Miquelle, D.G.; Peek, J.M.; Van Ballenberghe, V. Sexual segregation in Alaskan moose. Wildl. Monogr. 1992, 122, 1–57. [Google Scholar]
- Bowyer, R.T.; Pierce, B.M.; Duffy, L.K.; Haggstrom, D.A. Sexual segregation in moose: Effects of habitat manipulation. Alces 2001, 37, 109–122. [Google Scholar]
- Spaeth, D.F.; Bowyer, R.T.; Stephenson, T.R.; Barboza, P.S. Sexual segregation in moose Alces alces: An experimental manipulation of foraging behaviour. Wildl. Biol. 2004, 10, 59–72. [Google Scholar] [CrossRef]
- Oehlers, S.A.; Bowyer, R.T.; Huettmann, F.; Person, D.K.; Kessler, W.B. Sex and scale: Implications for habitat selection by Alaskan moose Alces alces gigas. Wildl. Biol. 2011, 17, 67–85. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, Y.; Li, J.; Kong, L.; Hu, H.; Pan, H.; Xu, L.; Deng, Y.; Li, Q.; Jin, L.; et al. Two Antarctic penguin genomes reveal insights into their evolutionary history and molecular changes related to the Antarctic environment. GigaScience 2014, 3, 27. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, L.; Chen, X.; Zhong, Y.; Yang, Y.; Xia, W.; Liu, C.; Zhu, W.; Wang, H.; Yan, B.; et al. Biological adaptations in the Arctic cervid, the reindeer (Rangifer tarandus). Science 2019, 364, eaav6312. [Google Scholar] [CrossRef]
- Renecker, L.A.; Hudson, R.J. Seasonal energy expenditures and thermoregulatory responses of moose. Can. J. Zool. 1986, 64, 322–327. [Google Scholar] [CrossRef]
- Thompson, D.P.; Barboza, P.S.; Crouse, J.A.; McDonough, T.J.; Badajos, O.H.; Herberg, A.M. Body temperature patterns vary with day, season, and body condition of moose (Alces alces). J. Mammal. 2019, 100, 1466–1478. [Google Scholar] [CrossRef]
- Melin, M.; Matala, J.; Mehtätalo, L.; Tiilikainen, R.; Tikkanen, O.-P.; Maltamo, M.; Pusenius, J.; Packalen, P. Moose (Alces alces) reacts to high summer temperatures by utilizing thermal shelters in boreal forests—An analysis based on airborne laser scanning of the canopy structure at moose locations. Glob. Chang. Biol. 2014, 20, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Jiang, G.; Stott, P.; Piao, R. Climate change impacts population dynamics and distribution shift of moose (Alces alces) in heilongjiang province of China. Ecol. Res. 2013, 28, 625–632. [Google Scholar] [CrossRef]
- Chen, S.; Holyoak, M.; Liu, H.; Bao, H.; Ma, Y.; Dou, H.; Jiang, G. Effects of Spatially Heterogeneous Warming on Gut Microbiota, Nutrition and Gene Flow of a Heat-sensitive Ungulate Population. Sci. Total Environ. 2021, 806, 150537. [Google Scholar] [CrossRef] [PubMed]
- Sand, G.C. Home-range size in relation to age and sex in moose. J. Mammal. 1994, 75, 1005–1012. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Linkie, M.; Ridout, M. Assessing tiger–prey interactions in Sumatran rainforests. J. Zool. 2011, 284, 224–229. [Google Scholar] [CrossRef]
- Rejecter, L.A.; Hudson, R.J. Behavioral and thermoregulatory responses of moose to high ambient temperatures and insect harassment in aspen dominated forests. Alces 1990, 6, 66–72. [Google Scholar]
- McCann, N.P.; Moen, R.A.; Harris, T.R. Warm-season heat stress in moose (Alces alces). Can. J. Zool. 2013, 91, 893–898. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.P. Evaluating the Physiological and Behavioral Responses of Moose (Alces alces) to Fluctuating Environmental Temperatures. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2020. [Google Scholar]
- Boyce, M.S. Scale for resource selection functions. Divers. Distrib. 2010, 12, 269–276. [Google Scholar] [CrossRef]
- Bowyer, R.T.; Kie, J.G. Effects of scale on interpreting life-history characteristics of ungulates and carnivores. Divers. Distrib. 2006, 12, 244–257. [Google Scholar] [CrossRef]
- Huettmann, F.; Diamond, A.W. Large-scale effects on the spatial distribution of seabirds in the Northwest Atlantic. Landsc. Ecol. 2006, 21, 1089–1108. [Google Scholar] [CrossRef]
- Zweifel, S.B.; Kreuzer, M.; Ewald, K.C.; Suter, W. Habitat selection by an alpine ungulate: The significance of forage characteristics varies with scale and season. Ecography 2009, 32, 103–113. [Google Scholar] [CrossRef]
- Godvik, I.M.R.; Loe, L.E.; Vik, J.O.; Veiberg, V.; Langvatn, R.; Mysterud, A. Temporal scales, trade-offs, and functional responses in red deer habitat selection. Ecology 2009, 90, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Herfindal, I.; Tremblay, J.-P.; Hansen, B.B.; Solberg, E.J.; Heim, M.; Sæther, B.-E. Scale dependency and functional response in moose habitat selection. Ecography 2009, 32, 849–859. [Google Scholar] [CrossRef]
- Jiang, G.; Ma, J.; Zang MScott, P. Multiple spatial-scale resource selection function models in relation to human disturbance for moose in northeastern China. Ecol. Res. 2009, 24, 423–440. [Google Scholar] [CrossRef]
- Van Beest, F.M.; Mysterud, A.; Loe, L.E.; Milner, J.M. Forage quantity, quality and depletion as scaledependent mechanisms drive habitat selection of a large browsing herbivore. J. Anim. Ecol. 2010, 79, 910–922. [Google Scholar] [PubMed]
- Sih, A. Optimal behavior: Can foragers balance two conflicting demands? Science 1980, 210, 1041–1043. [Google Scholar] [CrossRef]
- Beier, P.; McCullough, D.R. Factors influencing white-tailed deer activity patterns and habitat use. Wildl. Monogr. 1990, 109, 3–51. [Google Scholar]
- Anderson, M.; Mclellan, B.N.; Serrouya, R. Moose response to high-elevation forestry: Implications for apparent competition with endangered caribou. J. Wildl. Manag. 2018, 82, 299–309. [Google Scholar] [CrossRef]
- Barboza, P.S.; Bowyer, R.T. Sexual segregation in dimorphic deer: A new gastrocentric hypothesis. J. Mammal. 2000, 81, 473–489. [Google Scholar] [CrossRef]
- Bowyer, R.T. Sexual segregation in southern mule deer. J. Mammal. 1984, 65, 410–417. [Google Scholar] [CrossRef]
- Beier, P.; McCullough, D.R. Influence of sex and weather on white-tailed deer activity patterns. Appl. Anim. Behav. Sci. 1991, 29, 507–508. [Google Scholar] [CrossRef]
- Ferretti, F.; Fattorini, N. Competitor densities, habitat and weather: Effects on interspecific interactions between wild deer species. Integr. Zool. 2020, 16, 670–684. [Google Scholar] [CrossRef]
- Lowe, S.J.; Patterson, B.R.; Schaefer, J.A. Lack of behavioral responses of moose (Alces alces) to high ambient temperatures near the southern periphery of their range. Can. J. Zool. 2010, 88, 1032–1041. [Google Scholar] [CrossRef]
- Broders, H.G.; Coombs, A.B.; Mccarron, J.R. Ectothermic responses of moose (Alces alces) to thermoregulatory stress on mainland Nova Scotia. Alces 2012, 48, 53–61. [Google Scholar]
- Van Beest, F.M.; van Moorter, B.; Milner, J.M. Temperature-mediated habitat use and selection by a heat-sensitive northern ungulate. Anim. Behav. 2012, 84, 723–735. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Redilla, K.M.; Moll, R.J.; van Moorter, B.; Rolandsen, C.M.; Millspaugh, J.J.; Solberg, E.J. Movement modeling reveals the complex nature of the response of moose to ambient temperatures during summer. J. Mammal. 2019, 100, 169–177. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Ledee, O.E.; Thompson, L.M. Climate change effects on deer and moose in the midwest. J. Wildl. Manag. 2019, 83, 769–781. [Google Scholar] [CrossRef]
- Liu, S.; Qi, J.; Gu, J.; Long, Z.; Ma, J.; Jiang, G. What factors relate with the activity synchronization intensity among big cats and their ungulate prey in northeast china? Glob. Ecol. Conserv. 2021, 32, e01899. [Google Scholar] [CrossRef]
- Lenarz, M.S. 2008 Aerial Moose Survey. Minnesota Department of Natural Resources, St. Paul, USA. Available online: http://files.dnr.state.mn.us/outdoor_activities/hunting/moose/moose_survey_2008.pdf (accessed on 23 April 2008).
- Shively, R.; Crouse, J.; Thompson, D.; Barboza, P. Is summer food intake a limiting factor for boreal browsers? Diet, temperature, and reproduction as drivers of consumption in female moose. PLoS ONE 2019, 14, e0223617. [Google Scholar] [CrossRef] [PubMed]
- Borowik, T.; Ratkiewicz, M.; Malanko, W.; Duda, N.; Kowalczyk, R. Too hot to handle: Summer space use shift in a cold-adapted ungulate at the edge of its range. Landsc. Ecol. 2020, 35, 1341–1351. [Google Scholar] [CrossRef]
- Dussault, C.; Ouellet, J.-P.; Courtois, R.; Huot, J.; Breton, L.; LaRochelle, J. Behavioural responses of moose to thermal conditions in the boreal forest. Écoscience 2004, 11, 321–328. [Google Scholar] [CrossRef]
- Murray, D.L.; Cox, E.W.; Ballard, W.B.; Whitlaw, H.A.; Lenarz, M.S.; Custer, T.W.; Barnett, T.; Fuller, T.K. Pathogens, nutritional deficiency, and climate influences on a declining moose population. Wildl. Monogr. 2006, 166, 1–30. [Google Scholar] [CrossRef]
- Street, G.M.; Rodgers, A.R.; Fryxell, J.M. Mid-day temperature variation influences seasonal habitat selection by moose. J. Wildl. Manag. 2015, 79, 505–512. [Google Scholar] [CrossRef]
- Ditmer, M.A.; Moen, R.A.; Windels, S.K.; Forester, J.D.; Ness, T.E.; Harris, T.R. Moose at their bioclimatic edge alter their behavior based on weather, landscape, and predators. Curr. Zool. 2018, 64, 419–432. [Google Scholar] [CrossRef]






| Species | Parameter | Coef. | SE | Z Value | p | 95% CI | |
|---|---|---|---|---|---|---|---|
| Moose | Intercept | 13.02 | 1.37 | 9.50 | <0.01 | 10.42 | 15.81 |
| Elevation (800 m) | −0.01 | 0.00 | −8.71 | <0.01 | −0.02 | −0.01 | |
| Distance to larch forest (800 m) | 0.00 | 0.00 | 3.58 | <0.00 | 0.00 | 0.00 | |
| Abundance of Betula exilis shrub (3200 m) | 0.51 | 0.12 | 4.31 | <0.01 | 0.28 | 0.74 | |
| Herb coverage (3200 m) | −0.75 | 0.30 | −2.49 | <0.05 | −1.34 | −0.15 | |
| Roe deer | Intercept | 4.80 | 0.54 | 8.82 | <0.01 | 3.74 | 5.88 |
| Elevation (3200 m) | −0.00 | 0.00 | −7.13 | <0.01 | −0.01 | −0.00 | |
| Slope (200 m) | 0.06 | 0.01 | 4.24 | <0.01 | 0.03 | 0.09 | |
| Distance to larch forest (200 m) | 0.00 | 0.00 | 4.28 | <0.01 | 0.00 | 0.00 | |
| Abundance of Betula exilis shrub (1600 m) | −0.32 | 0.13 | −2.52 | <0.05 | −0.57 | −0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, H.; Zhai, P.; Wen, D.; Zhang, W.; Li, Y.; Yang, F.; Liang, X.; Yang, F.; Roberts, N.J.; Xu, Y.; et al. Effects of Inter- and Intra-Specific Interactions on Moose Habitat Selection Limited by Temperature. Remote Sens. 2022, 14, 6401. https://doi.org/10.3390/rs14246401
Bao H, Zhai P, Wen D, Zhang W, Li Y, Yang F, Liang X, Yang F, Roberts NJ, Xu Y, et al. Effects of Inter- and Intra-Specific Interactions on Moose Habitat Selection Limited by Temperature. Remote Sensing. 2022; 14(24):6401. https://doi.org/10.3390/rs14246401
Chicago/Turabian StyleBao, Heng, Penghui Zhai, Dusu Wen, Weihua Zhang, Ye Li, Feifei Yang, Xin Liang, Fan Yang, Nathan J. Roberts, Yanchun Xu, and et al. 2022. "Effects of Inter- and Intra-Specific Interactions on Moose Habitat Selection Limited by Temperature" Remote Sensing 14, no. 24: 6401. https://doi.org/10.3390/rs14246401
APA StyleBao, H., Zhai, P., Wen, D., Zhang, W., Li, Y., Yang, F., Liang, X., Yang, F., Roberts, N. J., Xu, Y., & Jiang, G. (2022). Effects of Inter- and Intra-Specific Interactions on Moose Habitat Selection Limited by Temperature. Remote Sensing, 14(24), 6401. https://doi.org/10.3390/rs14246401