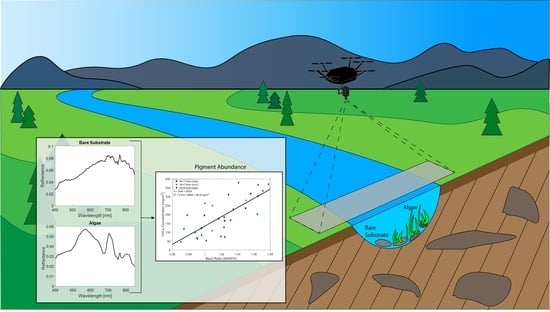
UAV-Based Hyperspectral Imaging for River Algae Pigment Estimation
Abstract
:1. Introduction
1.1. Background
1.2. Existing Methods
1.3. Ecological Context
1.4. Objectives
- present a framework for collecting high-spatial and -spectral image data using a UAV-based hyperspectral imaging system along a clear and shallow river;
- determine optimal linear relationships between spectral band ratios (i.e., the ratio of reflectance values at two wavelengths) and the algal pigments chl a and phycocyanin present among filamentous, epiphytic, and epilithic forms of benthic algae;
- compare the performance of the derived optimal linear relationships to select existing spectral indices developed for monitoring chl a.
2. Materials and Methods
2.1. Study Sites
2.2. RABs and Algal Phenology
2.3. Imaging System
2.3.1. Imager
2.3.2. UAV
2.4. Flight Overview
2.5. Ground-Truth Data Collection
2.6. Image Preprocessing
2.6.1. Image Calibration
2.6.2. Pixel Selection
2.7. Image Processing
2.7.1. Theoretical Basis for Isolating Stream Bed Reflectance
2.7.2. Development of Spectral Indices and Application to Algal Standing Crops
2.8. Uncertainty Analysis
3. Results
3.1. RAB Characteristics
3.2. Spectral Models
3.2.1. Separation by Growth Form
Filamentous plus Epiphytic
Epilithic
3.2.2. Separation by Phenology
Bear Gulch
- Total
Filamentous plus Epiphytic
Epilithic
Gold Creek
- Total
Filamentous plus Epiphytic
Epilithic
3.2.3. Results Summary
3.3. Generalizability of Band Ratios
3.4. Comparison of Brute-Force Method with Existing Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Watson, V.; Berlind, P.; Bahls, L. Control of Algal Standing Crop by P and N in the Clark Fork River. In Proceedings of the Clark Fork River Symposium, Missoula, MT, USA, 20–20 April 1990; pp. 47–62. [Google Scholar]
- Dodds, W.K.; Smith, V.H.; Zander, B. Developing nutrient targets to control benthic chlorophyll levels in streams: A case study of the Clark Fork River. Water Res. 1997, 31, 1738–1750. [Google Scholar] [CrossRef]
- Suplee, M.W.; Watson, V.; Dodds, W.K.; Shirley, C. Response Of Algal Biomass To Large-Scale Nutrient Controls In The Clark Fork River, Montana, United States. J. Am. Water Resour. Assoc. 2012, 48, 1008–1021. [Google Scholar] [CrossRef]
- Whitton, B.A. Biology of Cladophora in Freshwaters. Water Res. 1970, 4, 457–476. [Google Scholar] [CrossRef]
- Biggs, B.J.; Price, G.M. A survey of filamentous algal proliferations in new zealand rivers. N. Z. J. Mar. Freshw. Res. 1987, 21, 175–191. [Google Scholar] [CrossRef]
- Timoshkin, O.A.; Samsonov, D.P.; Yamamuro, M.; Moore, M.V.; Belykh, O.I.; Malnik, V.V.; Sakirko, M.V.; Shirokaya, A.A.; Bondarenko, N.A.; Domysheva, V.M.; et al. Rapid ecological change in the coastal zone of Lake Baikal (East Siberia): Is the site of the world’s greatest freshwater biodiversity in danger? J. Great Lakes Res. 2016, 42, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Whitman, R.L.; Shively, D.A.; Pawlik, H.; Nevers, M.B.; Byappanahalli, M.N. Occurrence of Escherichia coli and enterococci in Cladophora (Chlorophyta) in nearshore water and beach sand of Lake Michigan. Appl. Environ. Microbiol. 2003, 69, 4714–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhougstraete, M.P.; Byappanahalli, M.N.; Rose, J.B.; Whitman, R.L. Cladophora in the Great Lakes: Impacts on beach water quality and human health. Water Sci. Technol. 2010, 62, 68–76. [Google Scholar] [CrossRef]
- Heuvel, A.V.; McDermott, C.; Pillsbury, R.; Sandrin, T.; Kinzelman, J.; Ferguson, J.; Sadowsky, M.; Byappanahalli, M.; Whitman, R.; Kleinheinz, G.T. The Green Alga, Cladophora, Promotes Escherichia coli Growth and Contamination of Recreational Waters in Lake Michigan. J. Environ. Qual. 2010, 39, 333–344. [Google Scholar] [CrossRef]
- Montana Department of Environmantal Quality. 17.30.631 Numeric Algal Biomass And Nutrient Standards. 2002. Available online: https://rules.mt.gov/gateway/ruleno.asp?RN=17%2E30%2E631 (accessed on 31 May 2023).
- Richardson, L.L. Remote Sensing of Algal Bloom Dynamics. BioScience 1996, 46, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sengpiel, R.E.; Pascual, D.L.; Tedesco, L.P.; Wilson, J.S.; Soyeux, A. Using hyperspectral remote sensing to estimate chlorophyll-a and phycocyanin in a mesotrophic reservoir. Int. J. Remote Sens. 2010, 31, 4147–4162. [Google Scholar] [CrossRef]
- Steinman, A.D.; Lamberti, G.A.; Leavitt, P.R.; Uzarski, D.G. Biomass and Pigments of Benthic Algae. In Methods in Stream Ecology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 223–241. [Google Scholar]
- Topp, S.N.; Pavelsky, T.M.; Jensen, D.; Simard, M.; Ross, M.R. Research trends in the use of remote sensing for inland water quality science: Moving towards multidisciplinary applications. Water 2020, 12, 169. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, M.H.; Melesse, A.M.; Reddi, L. A comprehensive review on water quality parameters estimation using remote sensing techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.F.; Tang, D.L.; Wang, S. Distribution of chlorophyll and harmful algal blooms (HABs): A review on space based studies in the coastal environments of Chinese marginal seas. Adv. Space Res. 2008, 41, 12–19. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Cota, G.; Stuart, V.; Maass, H.; Platt, T. Remote sensing of phytoplankton pigments: A comparison of empirical and theoretical approaches. Int. J. Remote Sens. 2001, 22, 249–273. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Prieur, L.; Morel, A. A three-component model of ocean colour and its application to remote sensing of phytoplankton pigments in coastal waters. Int. J. Remote Sens. 1989, 10, 1373–1394. [Google Scholar] [CrossRef]
- Brando, V.E.; Dekker, A.G. Satellite hyperspectral remote sensing for estimating estuarine and coastal water quality. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1378–1387. [Google Scholar] [CrossRef]
- Ekstrand, S. Landsat TM based quantification of chlorophyll-a during algae blooms in coastal waters. Int. J. Remote Sens. 1992, 13, 1913–1926. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Robertson, L. An algorithm for detecting trophic status (chlorophyll-a), cyanobacterial-dominance, surface scums and floating vegetation in inland and coastal waters. Remote Sens. Environ. 2012, 124, 637–652. [Google Scholar] [CrossRef]
- Pahlevan, N.; Smith, B.; Binding, C.; Gurlin, D.; Li, L.; Bresciani, M.; Giardino, C. Hyperspectral retrievals of phytoplankton absorption and chlorophyll-a in inland and nearshore coastal waters. Remote Sens. Environ. 2021, 253, 112200. [Google Scholar] [CrossRef]
- Papenfus, M.; Schaeffer, B.; Pollard, A.I.; Loftin, K. Exploring the potential value of satellite remote sensing to monitor chlorophyll-a for US lakes and reservoirs. Environ. Monit. Assess. 2020, 192, 808. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.H.; Chan, S.N.; Lee, J.H. Remote sensing of coastal algal blooms using unmanned aerial vehicles (UAVs). Mar. Pollut. Bull. 2020, 152, 110889. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Pyo, J.C.; Kwon, Y.H.; Duan, H.; Cho, K.H.; Park, Y. Drone-based hyperspectral remote sensing of cyanobacteria using vertical cumulative pigment concentration in a deep reservoir. Remote Sens. Environ. 2020, 236. [Google Scholar] [CrossRef]
- Wu, D.; Li, R.; Zhang, F.; Liu, J. A review on drone-based harmful algae blooms monitoring. Environ. Monit. Assess. 2019, 191, 211. [Google Scholar] [CrossRef]
- King, T.; Hundt, S.; Hafen, K.; Stengel, V.; Ducar, S. Mapping the probability of freshwater algal blooms with various spectral indices and sources of training data. J. Appl. Remote Sens. 2022, 16, 044522. [Google Scholar] [CrossRef]
- Flynn, K.F.; Chapra, S.C. Remote sensing of submerged aquatic vegetation in a shallow non-turbid river using an unmanned aerial vehicle. Remote Sens. 2014, 6, 12815–12836. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Nam, S.H.; Koo, J.W.; Hwang, T.M. Hybrid approach of unmanned aerial vehicle and unmanned surface vehicle for assessment of chlorophyll-a imagery using spectral indices in stream, South Korea. Water 2021, 13, 1930. [Google Scholar] [CrossRef]
- Kupssinskü, L.S.; Guimarães, T.T.; De Souza, E.M.; Zanotta, D.C.; Veronez, M.R.; Gonzaga, L.; Mauad, F.F. A method for chlorophyll-a and suspended solids prediction through remote sensing and machine learning. Sensors 2020, 20, 2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kureel, N.; Sarup, J.; Matin, S.; Goswami, S.; Kureel, K. Modelling vegetation health and stress using hyperspectral remote sensing data. Model. Earth Syst. Environ. 2022, 8, 733–748. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Binding, C.; Greenberg, T.; Bukata, R. The MERIS Maximum Chlorophyll Index; its merits and limitations for inland water algal bloom monitoring. J. Great Lakes Res. 2013, 39, 100–107. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, D.; Zhuang, D.; Fu, J. Evaluation of hyperspectral indices for chlorophyll-a concentration estimation in Tangxun lake (Wuhan, China). Int. J. Environ. Res. Public Health 2010, 7, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.G.; Gray, L.J.; Grimm, N.B.; Busch, D.E. Temporal Succession in a Desert Stream Ecosystem Following Flash Flooding. Ecol. Monogr. 1982, 52, 93–110. [Google Scholar] [CrossRef]
- Minshall, G.W.; Petersen, R.C.; Cummins, K.W.; Bott, T.L.; Sedell, J.R.; Cushing, C.E.; Vannote, R.L. Interbiome comparison of stream ecosystem dynamics. Ecol. Monogr. 1983, 53, 1–25. [Google Scholar] [CrossRef]
- McClain, M.E.; Richey, J.E.; Brandes, J.A.; Pimentel, T.P. Dissolved organic matter and terrestrial-lotic linkages in the Central Amazon Basin of Brazil. Glob. Biogeochem. Cycles 1997, 11, 295–311. [Google Scholar] [CrossRef]
- Garcia, R.D.; Reissig, M.; Queimaliños, C.P.; Garcia, P.E.; Dieguez, M.C. Climate-driven terrestrial inputs in ultraoligotrophic mountain streams of Andean Patagonia revealed through chromophoric and fluorescent dissolved organic matter. Sci. Total Environ. 2015, 521, 280–292. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Thorp, J.H.; Delong, M.D. The Riverine Productivity Model: An Heuristic View of Carbon Sources and Organic Processing in Large River Ecosystems. Oikos 1994, 70, 305–308. [Google Scholar] [CrossRef]
- Tate, C.M. Patterns and controls of nitrogen in tallgrass prairie streams. Ecology 1990, 71, 2007–2018. [Google Scholar] [CrossRef]
- Valett, H.M.; Peipoch, M.; Poole, G.C. Nutrient processing domains: Spatial and temporal patterns of material retention in running waters. Freshw. Sci. 2022, 41, 195–214. [Google Scholar] [CrossRef]
- Minshall, G.W. Autotrophy in stream ecosystems. BioScience 1978, 28, 767–771. [Google Scholar] [CrossRef]
- Hall Jr, R.O.; Yackulic, C.B.; Kennedy, T.A.; Yard, M.D.; Rosi-Marshall, E.J.; Voichick, N.; Behn, K.E. Turbidity, light, temperature, and hydropeaking control primary productivity in the c olorado river, g rand c anyon. Limnol. Oceanogr. 2015, 60, 512–526. [Google Scholar] [CrossRef]
- Finlay, J.C. Patterns and controls of lotic algal stable carbon isotope ratios. Limnol. Oceanogr. 2004, 49, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, E.; Heffernan, J.; Grimm, N.; Stanley, E.; Harvey, J.; Arroita, M.; Appling, A.P.; Cohen, M.; McDowell, W.; Hall, R., Jr.; et al. The metabolic regimes of flowing waters. Limnol. Oceanogr. 2018, 63, S99–S118. [Google Scholar] [CrossRef] [Green Version]
- Battin, T.J.; Lauerwald, R.; Bernhardt, E.S.; Bertuzzo, E.; Gener, L.G.; Hall Jr, R.O.; Hotchkiss, E.R.; Maavara, T.; Pavelsky, T.M.; Ran, L.; et al. River ecosystem metabolism and carbon biogeochemistry in a changing world. Nature 2023, 613, 449–459. [Google Scholar] [CrossRef]
- Logan, R.D.; Hamp, S.M.; Torrey, M.A.; Feijo de Lima, R.; Colman, B.P.; Valett, H.; Shaw, J.A. UAV-Based Hyperspectral Imaging for River Algae Pigment Estimation and Development of a Low-Cost Multispectral Imager. In Proceedings of the SPIE Future Sensing Technologies Proceedings, Yokohoma, Japan, 22 May 2023. [Google Scholar]
- Ingman, G.L.; Kerr, M.A. Nutrient Sources in the Clark Fork River Basin. In Proceedings of the Clark Fork River Symposium, Missoula, MT, USA, 20–20 April 1990. [Google Scholar]
- Lohman, K.; Priscu, J.C. Physiological Indicators of Nutrient Deficiency in Cladophora Chlorophyta. J. Phycol. 1992, 28, 443–448. [Google Scholar] [CrossRef]
- Banish, N.J. Factors Influencing Cladophora Biomass Abundance in the Upper Clark Fork River, Montana. Ph.D Thesis, University of Montana, Missoula, MT, USA, 2017. [Google Scholar]
- Suplee, M.W.; Watson, V.; Teply, M.; McKee, H. How Green is Too Green? Public Opinion of What Constitutes Undesirable Algae Levels in Streams 1. JAWRA J. Am. Water Resour. Assoc. 2009, 45, 123–140. [Google Scholar] [CrossRef]
- Power, M.; Lowe, R.; Furey, P.; Welter, J.; Limm, M.; Finlay, J.; Bode, C.; Chang, S.; Goodrich, M.; Sculley, J. Algal mats and insect emergence in rivers under Mediterranean climates: Towards photogrammetric surveillance. Freshw. Biol. 2009, 54, 2101–2115. [Google Scholar] [CrossRef]
- Power, M.E.; Bouma-Gregson, K.; Higgins, P.; Carlson, S.M. The thirsty Eel: Summer and winter flow thresholds that tilt the Eel River of northwestern California from salmon-supporting to cyanobacterially degraded states. Copeia 2015, 103, 200–211. [Google Scholar] [CrossRef]
- Furey, P.C.; Lowe, R.L.; Power, M.E.; Campbell-Craven, A.M. Midges, Cladophora, and epiphytes: Shifting interactions through succession. Freshw. Sci. 2012, 31, 93–107. [Google Scholar] [CrossRef] [Green Version]
- DJI. Matrice 600 Pro—User Manual; Technical Report; DJI: Shenzhen, China, 2022. [Google Scholar]
- Resonon. Airborne User Manual; Technical Report; Resonon: Bozeman, MT, USA, 2022. [Google Scholar]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Moulton, T.P.; Souza, M.L.; Walter, T.L.; Krsulović, F.A. Patterns of periphyton chlorophyll and dry mass in a neotropical stream: A cheap and rapid analysis using a hand-held fluorometer. Mar. Freshw. Res. 2009, 60, 224–233. [Google Scholar] [CrossRef]
- Cremella, B.; Huot, Y.; Bonilla, S. Interpretation of total phytoplankton and cyanobacteria fluorescence from cross-calibrated fluorometers, including sensitivity to turbidity and colored dissolved organic matter. Limnol. Oceanogr. Methods 2018, 16, 881–894. [Google Scholar] [CrossRef]
- Legleiter, C.J.; Roberts, D.A.; Marcus, W.A.; Fonstad, M.A. Passive optical remote sensing of river channel morphology and in-stream habitat: Physical basis and feasibility. Remote Sens. Environ. 2004, 93, 493–510. [Google Scholar] [CrossRef] [Green Version]
- Legleiter, C.J.; Roberts, D.A.; Lawrence, R.L. Spectrally based remote sensing of river bathymetry. Earth Surf. Process. Landforms 2009, 34, 613–628. [Google Scholar] [CrossRef]
- Shaw, J.A. The effect of instrument polarization sensitivity on sea surface remote sensing with infrared spectroradiometers. J. Atmos. Ocean. Technol. 2002, 19, 820–827. [Google Scholar] [CrossRef]
- Logan, R.D.; Venkatesulu, E.; Shaw, J.A. Comparing the Polarimetric Response of Hyperspectral Imagers. In Proceedings of the Polarization Science and Remote Sensing X, San Diego, CA, USA, 1–5 August 2021; Volume 11833, pp. 90–97. [Google Scholar]
- Haltrin, V.I. Absorption and Scattering of Light in Natural Waters. In Light Scattering Reviews: Single and Multiple Light Scattering; Kokhanovsky, A.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 445–486. [Google Scholar] [CrossRef]
- Buiteveld, H.; Hakvoort, J.H.M.; Donze, M. Optical properties of pure water. Ocean. Opt. XII 1994, 2258, 174–183. [Google Scholar] [CrossRef]
- Ylöstalo, P.; Kallio, K.; Seppälä, J. Absorption properties of in-water constituents and their variation among various lake types in the boreal region. Remote Sens. Environ. 2014, 148, 190–205. [Google Scholar] [CrossRef]
- Karcz, D.; Boroń, B.; Matwijczuk, A.; Furso, J.; Staroń, J.; Ratuszna, A.; Fiedor, L. Lessons from chlorophylls: Modifications of porphyrinoids towards optimized solar energy conversion. Molecules 2014, 19, 15938–15954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, N.T.T.; Thao, N.T.P.; Koike, K.; Nhuan, M.T. Selecting the best band ratio to estimate chlorophyll-a concentration in a tropical freshwater lake using sentinel 2A images from a case study of Lake Ba Be (Northern Vietnam). ISPRS Int. J. Geo-Inf. 2017, 6, 290. [Google Scholar] [CrossRef] [Green Version]
- Shafique, N.A.; Fulk, F.; Autrey, B.C.; Flotemersch, J. Hyperspectral Remote Sensing of Water Quality Parameters for Large Rivers in the Ohio River Basin. In Proceedings of the 1st Interagency Conference on Research in the Watershed, Benson, AZ, USA, 27–30 October 2003; pp. 216–221. [Google Scholar]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Vermote, E.; Justice, C.; Claverie, M.; Franch, B. Preliminary analysis of the performance of the Landsat 8/OLI land surface reflectance product. Remote Sens. Environ. 2016, 185, 46–56. [Google Scholar] [CrossRef]
- Cho, H.J.; Kirui, P.; Natarajan, H. Test of Multi-spectral Vegetation Index for Floating and Canopy-forming Submerged Vegetation. Int. J. Environ. Res. Public Health 2008, 5, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Field Site | Field Date | Growth Form | chl a (mg/m2) | BOM (g FDM/m2) | Phycocyanin (μg/m2) |
|---|---|---|---|---|---|
| Gold Creek | 17 August | Filamentous | 158.9 ± 30.3 | 85.8 ± 13.3 | NA |
| Epiphytic | 7.3 ± 2.8 | 4.2 ± 0.4 | 6.5 ± 2.2 | ||
| Epilithic | 25.2 ± 2.2 | 8.8 ± 13.9 | 31.3 ± 4.6 | ||
| Total | 191.4 ± 33.1 | 97.4 ± 13.9 | 37.8 ± 4.6 | ||
| 9 September | Filamentous | 149.5 ± 24.0 | 67.4 ± 11.0 | NA | |
| Epiphytic | 10.0 ± 2.5 | 5.0 ± 1.7 | 7.2 ± 2.4 | ||
| Epilithic | 26.6 ± 3.8 | 9.9 ± 1.3 | 27.2 ± 4.6 | ||
| Total | 186.0 ± 30.3 | 82.4 ± 12.1 | 34.4 ± 5.5 | ||
| Bear Gulch | 17 August | Filamentous | 124.8 ± 25.8 | 55.5 ± 14.8 | NA |
| Epiphytic | 26.8 ± 9.9 | 17.2 ± 3.7 | 20.8 ± 5.2 | ||
| Epilithic | 31.9 ± 5.2 | 15.4 ± 2.5 | 21.7 ± 2.6 | ||
| Total | 183.5 ± 31.8 | 88.1 ± 18.1 | 42.5 ± 5.3 |
| Data Set | Growth Form | Optimal Band Ratio | ||
|---|---|---|---|---|
| Total | 684/674 | 0.57 | - | |
| Combined | Fila/epip | 684/674 | 0.62 | 0.05 |
| Epilithic | 729/809 | 0.44 | −0.13 | |
| Total | 554/536 | 0.95 | 0.38 | |
| Bear Gulch | Fila/epip | 554/536 | 0.96 | 0.39 |
| Epilithic | 573/589 | 0.86 | 0.29 | |
| Total | 684/674 | 0.63 | 0.06 | |
| Gold Creek | Fila/epip | 684/674 | 0.68 | 0.11 |
| Epilithic | 804/738 | 0.62 | 0.05 |
| Data Set | Growth Form | Optimal Band Ratio | ||
|---|---|---|---|---|
| Total | 753/824 | 0.41 | - | |
| Combined | Epiphytic | 688/655 | 0.33 | −0.08 |
| Epilithic | 738/804 | 0.50 | 0.09 | |
| Total | 625/619 | 0.92 | 0.51 | |
| Bear Gulch | Epiphytic | 748/740 | 0.94 | 0.53 |
| Epilithic | 525/519 | 0.89 | 0.48 | |
| Total | 804/748 | 0.46 | 0.05 | |
| Gold Creek | Epiphytic | 778/774 | 0.43 | 0.02 |
| Epilithic | 804/740 | 0.59 | 0.18 |
| Pigment | Growth Form | Optimal Band Ratio | |||
|---|---|---|---|---|---|
| Brute Force | Resampling | Brute Force | Resampling | ||
| Total | 684/674 | 684/674 | 0.57 | 0.57 ± 0.06 | |
| chl a | Fila/epip | 684/674 | 684/674 | 0.62 | 0.62 ± 0.05 |
| Epilithic | 729/809 | 729/809 | 0.44 | 0.44 ± 0.06 | |
| Total | 753/824 | 753/824 | 0.41 | 0.42 ± 0.09 | |
| Phycocyanin | Epiphytic | 688/655 | 684/674 | 0.33 | 0.59 ± 0.22 |
| Epilithic | 738/804 | 804/738 | 0.50 | 0.51 ± 0.06 | |
| Band Ratio | Center Wavelengths (nm) | Spectral Resolution (nm) | Regression Equation | p-Value | |
|---|---|---|---|---|---|
| Ha Index | 30, 35 | y = 177.4X − 61.3 | 0.12 | 0.048 | |
| Shafique Index | 5 | y = 154.5X − 47.4 | 0.13 | 0.041 | |
| NDVI | 30 | y = -235.5X + 125.8 | 0.04 | 0.25 | |
| Brute-Force Method | 2.1 | y = 2583X − 2448 | 0.57 | <0.001 | |
| Brute-Force Method, Normalized Difference | 2.1 | y = 5276X + 135.6 | 0.57 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logan, R.D.; Torrey, M.A.; Feijó-Lima, R.; Colman, B.P.; Valett, H.M.; Shaw, J.A. UAV-Based Hyperspectral Imaging for River Algae Pigment Estimation. Remote Sens. 2023, 15, 3148. https://doi.org/10.3390/rs15123148
Logan RD, Torrey MA, Feijó-Lima R, Colman BP, Valett HM, Shaw JA. UAV-Based Hyperspectral Imaging for River Algae Pigment Estimation. Remote Sensing. 2023; 15(12):3148. https://doi.org/10.3390/rs15123148
Chicago/Turabian StyleLogan, Riley D., Madison A. Torrey, Rafael Feijó-Lima, Benjamin P. Colman, H. Maurice Valett, and Joseph A. Shaw. 2023. "UAV-Based Hyperspectral Imaging for River Algae Pigment Estimation" Remote Sensing 15, no. 12: 3148. https://doi.org/10.3390/rs15123148
APA StyleLogan, R. D., Torrey, M. A., Feijó-Lima, R., Colman, B. P., Valett, H. M., & Shaw, J. A. (2023). UAV-Based Hyperspectral Imaging for River Algae Pigment Estimation. Remote Sensing, 15(12), 3148. https://doi.org/10.3390/rs15123148