Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Information
| Field | Year | Number of Samples | Area (ha) | N Rate (kg·ha−1) | Variety | Number of Leaves | Transplanting Date | Plant Density (hills·m−2) |
|---|---|---|---|---|---|---|---|---|
| F1 | 2011 | 33 | 29.6 | 97.9 | Kendao 6 | 12 | 17 May 2011 | 27 |
| F2 | 2011 | 4 | 13.1 | 105.9 | Longjing 26 | 11 | 20 May 2011 | 30 |
| F3 | 2011 | 4 | 31.0 | 101.0 | Kendao 6 | 12 | 12 May 2011 | 27 |
| F4 | 2012 | 14 | 10.7 | 120.2 | Longjing 31 | 11 | 16 May 2012 | 28 |
| F5 | 2012 | 37 | 21.6 | 98.3 | Longjing 31 | 11 | 20 May 2012 | 30 |
2.3. Remote Sensing Images and Preprocessing
2.4. Field Data Collection and Analysis
2.5. Data Analysis
| Vegetation Index | Formula | Ref. |
|---|---|---|
| Two-band vegetation indices | ||
| Ratio Vegetation Index 1 (RVI1) | NIR/B | [39] |
| Ratio Vegetation Index 2 (RVI2) | NIR/G | [40] |
| Ratio Vegetation Index 3 (RVI3) | NIR/R | [39] |
| Difference Index1 (DVI1) | NIR − B | [39] |
| Difference Index2 (DVI2) | NIR − G | [39] |
| Difference Index3 (DVI3) | NIR − R | [39] |
| Normalized Difference Vegetation Index 1 (NDVI1) | (NIR − R)/(NIR + R) | [40] |
| Normalized Difference Vegetation Index 2 (NDVI2) | (NIR − G)/(NIR + G) | [41] |
| Normalized Difference Vegetation Index 3 (NDVI3) | (NIR − B)/(NIR + B) | [40] |
| Renormalized Difference Vegetation Index 1 (RDVI1) | (NIR − B)/SQRT(NIR + B) | [42] |
| Renormalized Difference Vegetation Index 2 (RDVI2) | (NIR − G)/SQRT(NIR + G) | [42] |
| Renormalized Difference Vegetation Index 3 (RDVI3) | (NIR − R)/SQRT(NIR + R) | [42] |
| Chlorophyll Index (CI) | NIR/G − 1 | [43] |
| Wide Dynamic Range Vegetation Index 1 (WDRVI1) | (0.12 NIR − R)/(0.12∙NIR + R) | [44] |
| Wide Dynamic Range Vegetation Index 2 (WDRVI2) | (0.12 NIR − G)/(0.12∙NIR + G) | [44] |
| Wide Dynamic Range Vegetation Index 3 (WDRVI3) | (0.12 NIR − B)/(0.12∙NIR + B) | [44] |
| Soil Adjusted Vegetation Index (SAVI) | 1.5(NIR − R)/(NIR + R + 0.5) | [45] |
| Green Soil Adjusted Vegetation Index (GSAVI) | 1.5(NIR − G)/(NIR + G + 0.5) | [45] |
| Blue Soil Adjusted Vegetation Index (BSAVI) | 1.5(NIR − B)/(NIR + B + 0.5) | [45] |
| Modified Simple Ratio (MSR) | (NIR/R − 1)/SQRT(NIR/R + 1) | [46] |
| Optimal Soil Adjusted Vegetation Index (OSAVI) | (1 + 0.16)[(NIR − R)/(NIR + R + 0.16)] | [47] |
| Green Optimal Soil Adjusted Vegetation Index (GOSAVI) | (1 + 0.16)[(NIR − G)/(NIR + G + 0.16)] | [47] |
| Blue Optimal Soil Adjusted Vegetation Index (BOSAVI) | (1 + 0.16)[(NIR − B)/(NIR + B + 0.16)] | [47] |
| Modified Soil Adjusted Vegetation Index (MSAVI) | 0.5{2∙NIR + 1 − SQRT[(2∙NIR + 1)2 − 8(NIR − R)]} | [48] |
| Modified Green Soil Adjusted Vegetation Index (MGSAVI1) | 0.5{2∙NIR + 1 − SQRT[(2∙NIR + 1)2 − 8(NIR − G)]} | [48] |
| Modified Blue Soil Adjusted Vegetation Index (MBSAVI) | 0.5{2∙NIR + 1 − SQRT[(2∙NIR + 1)2 − 8(NIR − B)]} | [48] |
| Three-band vegetation indices | ||
| Simple Ratio Vegetation Index (SR) | R/G × NIR | [49] |
| Modified Normalized Difference Vegetation Index 1 (mNDVI1) | (NIR − R + 2∙G)/(NIR + R − 2∙G) | [50] |
| Modified Normalized Difference Vegetation Index 2 (mNDVI2) | (NIR − R + 2∙B)/(NIR + R − 2∙B) | [50] |
| New Modified Simple Ratio (mSR) | (NIR − B)/(R − B) | [51] |
| Visible Atmospherically-Resistant Index (VARI) | (G − R)/(G + R − B) | [52] |
| Structure Insensitive Pigment Index (SIPI) | (NIR − B)/(NIR − R) | [53] |
| Structure Insensitive Pigment Index 1 (SIPI1) | (NIR − B)/(NIR − G) | [53] |
| Normalized Different Index (NDI) | (NIR − R)/(NIR − G) | [49] |
| Plant Senescence Reflectance Index (PSRI) | (R − B)/NIR | [51] |
| Plant Senescence Reflectance Index 1 (PSRI1) | (R − G)/NIR | [51] |
| Modified Chlorophyll Absorption in Reflectance Index (MCARI) | [(NIR − R) − 0.2(R − G)] × (NIR/R) | [54] |
| Modified Chlorophyll Absorption in Reflectance Index 1 (MCARI1) | 1.2[2.5(NIR − R) − 1.3(NIR − G)] | [55] |
| Modified Chlorophyll Absorption in Reflectance Index 2 (MCARI2) | 1.2[2.5(NIR − R) − 1.3(R − G)]/SQRT[(2∙NIR + 1)2 − (6∙NIR − 5∙SQRT(R) − 0.5] | [55] |
| Triangular Vegetation Index (TVI) | 0.5[120(NIR − G) − 200(R − G)] | [57] |
| Modified Triangular Vegetation Index 1 (MTVI1) | 1.2[1.2(NIR − G) − 2.5(R − G)] | [55] |
| Modified Triangular Vegetation Index 2 (MTVI2) | 1.5[1.2(NIR − G) − 2.5(R − G)]/SQRT[(2∙NIR + 1)2 − (6∙NIR − 5∙SQRT(R) − 0.5] | [55] |
| Modified Triangular Vegetation Index 3 (MTVI3) | 1.5[1.2(NIR − B) − 2.5(R − B)]/ SQRT[(2 NIR + 1)2 − (6 NIR − 5 SQRT(R) − 0.5] | [55] |
| Enhanced Vegetation Index (EVI) | 2.5(NIR − R)/(1 + NIR + 6 R − 7.5 B) | [58] |
| Transformed Chlorophyll Absorption in Reflectance Index (TCARI) | 3[(NIR − R) − 0.2(NIR − G)(NIR/R)] | [56] |
| Triangular Chlorophyll Index (TCI) | 1.2(NIR − G) − 5(R − G)(NIR/R)^0.5 | [59] |
| TCARI/OSAVI | TCARI/OSAVI | [56] |
| MCARI/MTVI2 | MCARI/MTVI2 | [60] |
| TCARI/MSAVI | TCARI/MSAVI | [56] |
| TCI/OSAVI | TCI/OSAVI | [59] |
2.6. The Estimation of NNI
3. Results
3.1. Variability of Rice N Status Indicators
| Mean | Minimum | Maximum | SD | CV (%) | |
|---|---|---|---|---|---|
| 2011 | |||||
| Biomass (t·ha−1) | 0.87 | 0.50 | 1.55 | 0.22 | 25 |
| Leaf Area Index | 0.84 | 0.52 | 1.51 | 0.20 | 23 |
| Plant N concentration (%) | 2.76 | 2.45 | 3.06 | 0.14 | 5 |
| SPAD value | 42.30 | 37.03 | 44.08 | 1.80 | 4 |
| Plant N uptake (kg·ha−1) | 23.86 | 12.97 | 43.25 | 5.80 | 24 |
| Nitrogen Nutrition Index | 1.01 | 0.89 | 1.17 | 0.05 | 5 |
| 2012 | |||||
| Biomass (t·ha−1) | 2.91 | 1.45 | 4.68 | 0.79 | 27 |
| Leaf Area Index | 3.34 | 1.77 | 5.66 | 0.86 | 26 |
| Plant N concentration (%) | 2.24 | 1.75 | 2.77 | 0.25 | 11 |
| SPAD Value | 40.60 | 37.07 | 43.40 | 1.68 | 4 |
| Plant N uptake (kg·ha−1) | 65.00 | 30.11 | 114.9 | 17.93 | 28 |
| Nitrogen Nutrition Index | 1.15 | 0.83 | 1.50 | 0.16 | 14 |
| Field | Biomass (t·ha−1) | Plant N Concentration (%) | SPAD Value | NNI |
|---|---|---|---|---|
| F1 | 0.81 ± 0.16 | 2.77 ± 0.14 | 43.07 ± 0.62 | 1.00 ± 0.05 |
| F2 | 1.27 ± 0.25 | 2.63 ± 0.14 | 37.89 ± 0.89 | 1.03 ± 0.10 |
| F3 | 0.97 ± 0.17 | 2.62 ± 0.11 | 39.83 ± 0.65 | 1.00 ± 0.04 |
| F4 | 3.89 ± 0.41 | 2.12 ± 0.28 | 40.90 ± 1.08 | 1.21 ± 0.16 |
| F5 | 2.53 ± 0.53 | 2.29 ± 0.23 | 40.49 ± 1.85 | 1.13 ± 0.16 |
3.2. Vegetation Index Analysis
| Index | 2011 | 2012 | 2011 + 2012 | Index | 2011 | 2012 | 2011 + 2012 |
|---|---|---|---|---|---|---|---|
| Aboveground Biomass (t·ha−1) | LAI | ||||||
| MCARI | 0.67 ** | 0.62 ** | 0.90 ** | MCARI | 0.67 ** | 0.58 ** | 0.90 ** |
| DVI3 | 0.65 ** | 0.63 ** | 0.90 ** | DVI2 | 0.67 ** | 0.58 ** | 0.91 ** |
| TVI | 0.64 ** | 0.64 ** | 0.90 ** | RVI3 | 0.65 ** | 0.60 ** | 0.90 ** |
| RVI3 | 0.64 ** | 0.63 ** | 0.90 ** | DVI3 | 0.65 ** | 0.60 ** | 0.91 ** |
| MTVI1 | 0.63 ** | 0.64 ** | 0.90 ** | RDVI2 | 0.65 ** | 0.58 ** | 0.90 ** |
| MCARI1 | 0.63 ** | 0.64 ** | 0.90 ** | WDRVI1 | 0.65 ** | 0.60 ** | 0.90 ** |
| TCARI | 0.63 ** | 0.64 ** | 0.89 ** | MSR | 0.65 ** | 0.60 ** | 0.90 ** |
| WDRVI1 | 0.63 ** | 0.64 ** | 0.89 ** | RDVI3 | 0.64 ** | 0.60 ** | 0.90 ** |
| MSR | 0.63 ** | 0.64 ** | 0.90 ** | SAVI | 0.63 ** | 0.61 ** | 0.88 ** |
| SAVI | 0.61 ** | 0.64 ** | 0.87 ** | NDVI1 | 0.63 ** | 0.61 ** | 0.88 ** |
| Plant N Concentration (%) | SPAD Values | ||||||
| DVI4 | 0.55 ** | TCI | 0.27 ** | 0.17 ** | 0.13 ** | ||
| RDVI4 | 0.53 ** | PSRI | 0.19 ** | 0.10 ** | |||
| NDVI4 | 0.49 ** | MTVI2 | 0.18 ** | 0.22 ** | 0.16 ** | ||
| RDVI2 | 0.49 ** | TCARI | 0.16 ** | 0.22 ** | 0.14 ** | ||
| RVI4 | 0.49 ** | MCARI2 | 0.15 * | 0.23 ** | 0.15 ** | ||
| MGSAVI | 0.48 ** | WDRVI1 | 0.14 * | 0.20 ** | 0.12 ** | ||
| NDVI2 | 0.48 ** | MTVI3 | 0.10 * | 0.25 ** | 0.13 ** | ||
| GOSAVI | 0.48 ** | TCARI/OSAVI | 0.14 ** | ||||
| WDRVI2 | 0.47 ** | EVI | 0.14 ** | ||||
| mNDVI1 | 0.30 ** | DVI | 0.13* | 0.19 | |||
| Plant N Uptake (kg·ha−1) | NNI | ||||||
| RVI3 | 0.66 ** | 0.61 ** | 0.87 ** | RDVI1 | 0.18 ** | 0.32 ** | 0.41 ** |
| TVI | 0.66 ** | 0.61 ** | 0.87 ** | DVI2 | 0.17 ** | 0.33 ** | 0.43 ** |
| WDRVI1 | 0.66 ** | 0.62 ** | 0.87 ** | RVI2 | 0.17 ** | 0.33 ** | 0.44 ** |
| RDVI3 | 0.66 ** | 0.62 ** | 0.87 ** | WDRVI2 | 0.16 ** | 0.34 ** | 0.43 ** |
| TCARI | 0.65 ** | 0.63 ** | 0.86 ** | DVI3 | 0.16 ** | 0.34 ** | 0.43 ** |
| MSR | 0.65 ** | 0.62 ** | 0.87 ** | RDVI2 | 0.16 ** | 0.34 ** | 0.42 ** |
| MCARI1 | 0.65 ** | 0.62 ** | 0.87 ** | RVI3 | 0.16 ** | 0.34 ** | 0.45 ** |
| MTVI1 | 0.65 ** | 0.62 ** | 0.87 ** | WDRVI1 | 0.15 * | 0.35 ** | 0.44 ** |
| SAVI | 0.64 ** | 0.62 ** | 0.85 ** | RDVI3 | 0.15 * | 0.35 ** | 0.43 ** |
| OSAVI | 0.64 ** | 0.62 ** | 0.85 ** | TVI | 0.15 * | 0.34 ** | 0.44 ** |

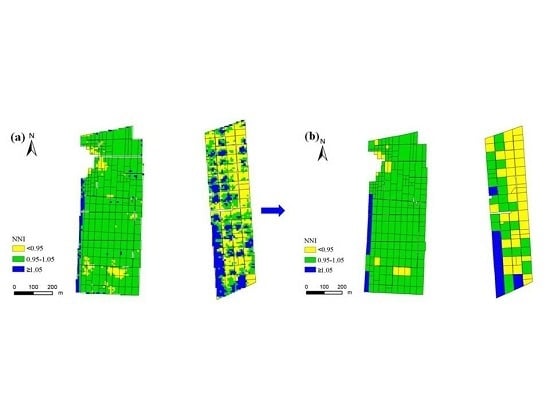
3.3. Nitrogen Status Diagnosis




4. Discussion
4.1. Direct Estimation of NNI
4.2. Indirect Estimation of NNI
4.3. Applications for Rice N Status Diagnosis and Topdressing N Recommendation
4.4. Challenges and Future Research Needs
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Dawe, D. The contribution of rice research to poverty alleviation. Stud. Plant Sci. 2000, 7, 3–12. [Google Scholar]
- Zhang, F.; Cui, Z.; Fan, M.; Zhang, W.; Chen, X.; Jiang, R. Integrated soil-crop system management: Reducing environmental risk while increasing crop productivity and improving nutrient use efficiency in China. J. Environ. Qual. 2011, 40, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Zhang, W.; Cui, Z.; Ma, W.; Chen, X.; Jiang, R. Nutrient use efficiencies of major cereal crops in China and measures for improvement. Acta Pedolog. Sin. 2008, 5, 915–924. [Google Scholar]
- Jin, J. Changes in the efficiency of fertiliser use in China. J. Sci. Food Agric. 2012, 92, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Xing, G.; Chen, X.; Zhang, S.; Zhang, L.; Liu, X.; Cui, Z.; Yin, B.; Christie, P.; Zhu, Z.; et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA. 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doberman, A.; Witt, C.; Dawe, D.; Abdulrachman, S.; Gines, H.C.; Nagarajan, R.; Satawathananont, S.; Son, T.T.; Tan, P.S.; Wang, G.H.; et al. Site-specific nutrient management for intensive rice cropping systems in Asia. Field Crops Res. 2002, 74, 37–66. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Neeteson, J.J.; Draycott, A. Quantitative relationships for the dependence of growth rate of arable crops on their nitrogen content, dry weight and aerial environment. Plant Soil 1986, 91, 281–301. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Gastal, F.; Lemaire, G.; Draycott, A.; Millard, P.; Neeteson, J.J. Growth rate and % N of field grown crops: Theory and experiments. Ann. Bot. 1991, 67, 181–190. [Google Scholar]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Houlès, V.; Guerif, M.; Mary, B. Elaboration of a nitrogen nutrition indicator for winter wheat based on leaf area index and chlorophyll content for making nitrogen recommendations. Eur. J. Agron. 2007, 27, 1–11. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with crop circle multispectral active canopy sensor. Field Crops Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, Y.; Cao, Q.; Wang, H.; Gnyp, M.L.; Bareth, G.; Khosla, R.; Yang, W.; Liu, F.; Liu, C. In-season estimation of rice nitrogen status with an active crop canopy sensor. IEEE J. Sel. Topics Appl. Earth Observ. Remote Sens. 2014, 7, 4403–4413. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between the normalized SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Prost, L.; Jeuffroy, M.H. Replacing the nitrogen nutrition index by the chlorophyll meter to assess wheat N status. Agron. Sustain. Dev. 2007, 27, 321–330. [Google Scholar] [CrossRef]
- Ziadi, N.; Bélanger, G.; Claessens, A.; Lefebvre, L.; Tremblay, N.; Cambouris, A.N.; Nolin, M.C.; Parent, L.É. Plant-based diagnostic tools for evaluating wheat nitrogen status. Crop Sci. 2010, 50, 2580–2590. [Google Scholar] [CrossRef]
- Cao, Q.; Cui, Z.; Chen, X.; Khosla, R.; Dao, T.H.; Miao, Y. Quantifying spatial variability of indigeneous nitrogen supply for precision nitrogen management in small sacle farming. Precis. Agric. 2012, 13, 45–61. [Google Scholar] [CrossRef]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Claessens, A.; Tremblay, N.; Cambouris, A.N.; Nolin, M.C.; Parent, L.-É. Chlorophyll measurements and nitrogen nutrition index for the evaluation of corn nitrogen status. Agron. J. 2008, 100, 1264–1273. [Google Scholar] [CrossRef]
- Miao, Y.; Mulla, D.J.; Randall, G.W.; Vetsch, J.A.; Vintila, R. Combining chlorophyll meter readings and high spatial resolution remote sensing images for in-season site-specific nitrogen management of corn. Precis. Agric. 2009, 10, 45–62. [Google Scholar] [CrossRef]
- Mistele, B.; Schmidhalter, U. Estimating the nitrogen nutrition index using spectral canopy reflectance measurements. Eur. J. Agron. 2008, 29, 184–190. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J.; Huang, W.; Tremblay, N.; Ouyang, Z.; Zhang, Q. Critical nitrogen curve and remote detection of nitrogen nutrition index for corn in the Northwestern Plain of Shandong Province, China. IEEE J. Sel. Topics Appl. Earth Observ. Remote Sens. 2013, 6, 682–689. [Google Scholar] [CrossRef]
- Holland, K.H.; Lamb, D.W.; Schepers, J.S. Radiometry of proximal active optical sensors (AOS) for agricultural sensing. IEEE J. Sel. Topics Appl. Earth Observ. Remote Sens. 2012, 5, 1793–1802. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Cilia, C.; Panigada, C.; Rossini, M.; Meroni, M.; Busetto, L.; Amaducci, S.; Boschetti, M.; Picchi, V.; Colombo, R. Nitrogen status assessment for variable rate fertilization in maize through hyperspectral imagery. Remote Sens. 2014, 6, 6549–6565. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, D.; Rosen, C.J.; Bauer, M.E. Comparison of petiole nitrate concentrations, SPAD chlorophyll readings, and QuickBird satellite imagery in detecting nitrogen status of potato canopies. Field Crops Res. 2007, 101, 96–103. [Google Scholar] [CrossRef]
- Yang, C.M.; Liu, C.C.; Wang, Y.W. Using FORMOSAT-2 satellite data to estimate leaf area index of rice crop. J. Photogram. Remote Sens. 2008, 13, 253–260. [Google Scholar]
- Darvishzadeh, R.; Matkan, A.A.; Ahangar, A.D. Inversion of a radiative transfer model for estimation of rice canopy chlorophyll content using a lookup-table approach. IEEE J. Sel. Topics Appl. Earth Observ. Remote Sens. 2012, 5, 1222–1230. [Google Scholar] [CrossRef]
- Xing, B.; Dudas, M.J.; Zhang, Z.; Qi, X. Pedogenetic characteristics of albic soils in the three river plain, Heilongjiang Province. Acta Pedolog. Sin. 1994, 31, 95–104. [Google Scholar]
- Wang, Y.; Yang, Y. Effects of agriculture reclamation on hydrologic characteristics in the Sanjiang Plain. Chin. Geogr. Sci. 2001, 11, 163–167. [Google Scholar] [CrossRef]
- Yan, M.; Deng, W.; Chen, P. Climate change in the Sanjiang Plain disturbed by large-scale reclamation. J. Geogr. Sci. 2002, 12, 405–412. [Google Scholar]
- Yao, Y.; Miao, Y.; Huang, S.; Gao, L.; Zhao, G.; Jiang, R.; Chen, X.; Zhang, F.; Yu, K.; et al. Active canopy sensor-based precision N management strategy for rice. Agron. Sustain. Dev. 2012, 32, 925–933. [Google Scholar] [CrossRef]
- Zhao, G.; Miao, Y.; Wang, H.; Su, M.; Fan, M.; Zhang, F.; Jiang, R.; Zhang, Z.; Liu, C.; Liu, P.; et al. A preliminary precision rice management system for increasing both grain yield and nitrogen use efficiency. Field Crops Res. 2013, 154, 23–30. [Google Scholar] [CrossRef]
- Chern, J.S.; Wu, A.M.; Lin, S.F. Lesson learned from FORMOSAT-2 mission operations. Acta Astronaut. 2006, 59, 344–350. [Google Scholar] [CrossRef]
- Liu, C.C. Processing of FORMOSAT-2 daily revisit imagery for site surveillance. IEEE Trans. Geosci. Remote Sens. 2006, 44, 3206–3214. [Google Scholar] [CrossRef]
- Bei, J.H.; Wang, K.R.; Chu, Z.D.; Chen, B.; Li, S.K. Comparitive study on the measurement methods of the leaf area. J. Shihezi Uni. 2005, 23, 216–218. [Google Scholar]
- Lv, W.; Ge, Y.; Wu, J.; Chang, J. Study on the method for the determination of nitric nitrogen, ammoniacal nitrogen and total nitrogen in plant. Spectrosc. Spect. Anal. 2004, 24, 204–206. [Google Scholar]
- Li, N. The comparison on various methods for determing different proteins. J. Shanxi Agric. Univ. 2006, 26, 132–134. [Google Scholar]
- Justes, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelier-Huche, L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Datt, B. Visible/near infrared reflectance and chlorophyll content in Eucalyptus leaves. Int. J. Remote Sens. 1999, 20, 2741–2759. [Google Scholar] [CrossRef]
- Wang, W.; Yao, X.; Yao, X.F.; Tian, Y.C.; Liu, X.J.; Ni, J.; Cao, W.X.; Zhu, Y. Estimating leaf nitrogen concentration with three-band vegetation indices in rice and wheat. Field Crops Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stage. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Peñuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica. 1995, 32, 221–230. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Colstoun, E.B.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote estimation of crop chlorophyll content using spectral indices derived from hyperspectral data. IEEE Trans.Geosci. Remote. Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Long, D.S.; Gessler, P.E.; Smith, A.M.S. Using in-situ measurements to evaluate the new RapidEye™ satellite series for prediction of wheat nitrogen status. Int. J. Remote Sens. 2007, 28, 4183–4190. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crops Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Yu, K.; Li, F.; Gnyp, M.L.; Miao, Y.; Bareth, G.; Chen, X. Remotely detecting canopy nitrogen concentration and uptake of paddy rice in the Northeast China Plain. ISPRS J. Photogramm. Remote Sens. 2013, 78, 102–115. [Google Scholar] [CrossRef]
- Van Niel, T.G.; McVicar, T.R. Current and potential uses of optical remote sensing in rice-based irrigation systems: A review. Aust. J. Agric. Res. 2004, 55, 155–185. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q.; Cui, K.; Zhang, F.; Dobermann, A. Improving nitrogen fertilization in rice by site-specific N management. A review. Agron. Sustain. Dev. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Shen, J.; Cui, Z.; Miao, Y.; Mi, G.; Zhang, H.; Fan, M.; Zhang, C.; Jiang, R.; Zhang, W.; Li, H.; et al. Transforming agriculture in China: From solely high yield to both high yield and high resource use efficiency. Global Food Sec. 2013, 2, 1–8. [Google Scholar] [CrossRef]
- Koppe, W.; Gnyp, M.L.; Hütt, C.; Yao, Y.; Miao, Y.; Chen, X.; Bareth, B. Rice monitoring with multi-temporal and dual-polarimetric TerraSAR-X data. Int. J. Appl. Earth Observ. Geoinf. 2013, 21, 568–576. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agricuture: A review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Huang, Y.; Thomson, S.J.; Hoffmann, W.C.; Lan, Y.; Fritz, B.K. Development and prospect of unmanned aerial vehicle technologies for agricultural production management. Int. J. Agric. Biol. Eng. 2013, 6, 1–10. [Google Scholar]
- Uto, K.; Seki, H.; Saito, G.; Kosugi, Y. Characterization of rice paddies by a UAV-mounted miniature hyperspectral sensor system. IEEE J. Sel. Topics Appl. Earth Observ. Remote Sens. 2013, 6, 851–860. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crops Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.L.; Lenz-Wiedemann, V.I.S.; Rascher, U.; et al. Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China. Remote Sens. 2015, 7, 10646-10667. https://doi.org/10.3390/rs70810646
Huang S, Miao Y, Zhao G, Yuan F, Ma X, Tan C, Yu W, Gnyp ML, Lenz-Wiedemann VIS, Rascher U, et al. Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China. Remote Sensing. 2015; 7(8):10646-10667. https://doi.org/10.3390/rs70810646
Chicago/Turabian StyleHuang, Shanyu, Yuxin Miao, Guangming Zhao, Fei Yuan, Xiaobo Ma, Chuanxiang Tan, Weifeng Yu, Martin L. Gnyp, Victoria I.S. Lenz-Wiedemann, Uwe Rascher, and et al. 2015. "Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China" Remote Sensing 7, no. 8: 10646-10667. https://doi.org/10.3390/rs70810646
APA StyleHuang, S., Miao, Y., Zhao, G., Yuan, F., Ma, X., Tan, C., Yu, W., Gnyp, M. L., Lenz-Wiedemann, V. I. S., Rascher, U., & Bareth, G. (2015). Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China. Remote Sensing, 7(8), 10646-10667. https://doi.org/10.3390/rs70810646