Quantifying Fertilizer Application Response Variability with VHR Satellite NDVI Time Series in a Rainfed Smallholder Cropping System of Mali
Abstract
:1. Introduction
2. Data Acquisition and Processing
2.1. Study Area, Near Sukumba, Mali
2.2. VHR Satellite Series
- Ortho ready DG-products with no DEM correction by DG (base elevation) were orthorectified using SRTM 30 m DEM and GCPs when available, i.e., after 26 August 2014. Out of the total set of 65 GCPs, a subset of 38 GCPs was used to correct the 18 October 2014 image. Orthorectification resulted in a geometric accuracy of 0.84 m (RMSE), assessed on remaining GCPs not used for orthorectification. This image was thereafter taken as master image to geometrically correct the other images in the absence of GCPs (methods 2 and 4 in Table 1). The same set of GCPs was used to orthorectify the 3 last ortho ready standard images of the series using the same method.
- Ortho ready DG-products acquired before 26 August 2014 were first orthorectified with the SRTM 30 m DEM without any GCP, and were subsequently co-registered to the master image (i.e., 18 October 2014 image orthorectified with the first method as describe here above) thanks to an automatic image-to-image registration algorithm providing 25 tie-points well distributed over the image.
- Products orthorectified by DG are of two types: standard product orthorectified using a coarse DEM (SRTM 90 m) and orthorectified product pre-processed by DG with fine DEM (SRTM 30 m). Both product types were simply georeferenced with the set of 38 GCPs for dates after 26 August 2014.
- Products orthorectified by DG (both standard product and orthorectified product types) acquired before 26 August 2014 were georeferenced using the master image by automatic image-to-image registration (as mentioned above for case 2).
2.3. In-Situ Data Collection
2.3.1. Soil Fertility Trials
2.3.2. Crop Development
2.3.3. Farming Practices
2.4. UAV Data Acquisition and Processing
3. Methods
3.1. Seasonal NDVI Profiles under Different Fertilizer Applications
3.2. Detection of Fertilizer Application Responses at the Field Scale
3.3. Relationships between Plant Growth Indicators and NDVI
3.4. Detection of Crops Fertilization Treatment at Landscape Level
4. Results and Discussion
4.1. Seasonal NDVI Profiles under Different Fertilization Levels
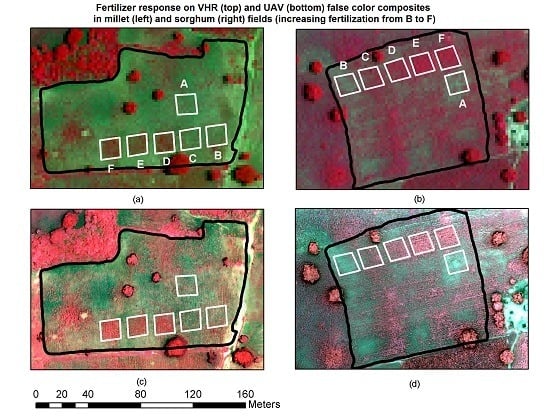
4.2. Detection of Fertilizer Applications at the Field Scale
4.3. Relationships between Plant Growth Indicators and NDVI
4.4. Detection of Crops Fertilization Treatment at Landscape Level
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Godfray, H.C.; Garnett, T. Food security and sustainable intensification. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20120273. [Google Scholar] [CrossRef] [PubMed]
- Van Ittersum, M.K.; Cassman, K.G.; Grassini, P.; Wolf, J.; Tittonell, P.; Hochman, Z. Yield gap analysis with local to global relevance—A review. Field Crops Res. 2013, 143, 4–17. [Google Scholar] [CrossRef]
- Panel, T.M. Sustainable Intensification: A New Paradigm for African Agriculture; Imperial College London: London, UK, 2013. [Google Scholar]
- Padgham, J.; Abubakari, A.; Ayivor, J.; Dietrich, K.; Fosu-Mensah, B.; Gordon, C.; Habtezion, S.; Lawson, E.; Mensah, A.; Nukpezah, D.; et al. Vulnerability and Adaptation to Climate Change in Semi-Arid Areas in West Africa: ASSAR Regional Diagnostic Study. Available online: https://www.weadapt.org/knowledge-base/assar/vulnerability-and-adaptation-to-climate-change-in-the-semi-arid-regions-of-east-africa (accessed on 20 June 2016).
- Giller, K.E.; Tittonell, P.; Rufino, M.C.; van Wijk, M.T.; Zingore, S.; Mapfumo, P.; Adjei-Nsiah, S.; Herrero, M.; Chikowo, R.; Corbeels, M.; et al. Communicating complexity: Integrated assessment of trade-offs concerning soil fertility management within African farming systems to support innovation and development. Agric. Syst. 2011, 104, 191–203. [Google Scholar] [CrossRef]
- Bielders, C.L.; Gérard, B. Millet response to microdose fertilization in south-western Niger: Effect of antecedent fertility management and environmental factors. Field Crops Res. 2015, 171, 165–175. [Google Scholar] [CrossRef]
- Soumaré, M. Cotton-Based Cropping Systems Dynamics and Sustainability in MALI; Université Paris X Nanterre: Nanterre, France, 2008. [Google Scholar]
- Gobin, A.; Campling, P.; Deckers, J.; Feyen, J. Integrated toposequence analyses to combine local and scientific knowledge systems. Geoderma 2000, 97, 103–123. [Google Scholar] [CrossRef]
- Gandah, M.; Brouwer, J.; Hiernaux, P.; Van Duivenbooden, N. Fertility management and landscape position: Farmers’ use of nutrient sources in western Niger and possible improvements. Nutr. Cycl. Agroecosyst. 2003, 67, 55–66. [Google Scholar] [CrossRef]
- Stoop, W.A. Variations in soil properties along three toposequences in Burkina Faso and implications for the development of improved cropping systems. Agric. Ecosyst. Environ. 1987, 19, 241–264. [Google Scholar] [CrossRef]
- Bazile, D.; Dembele, S.; Soumare, M.; Dembele, D. Utilisation de la diversite varietale du sorgho pour valoriser la diversite des sols au Mali. Cah. Agric. 2008, 17, 86–94. [Google Scholar]
- Traore, K.; Ganry, F.; Oliver, R.; Gigou, J. Litter production and soil fertility in a Vitellaria paradoxa parkland in a catena in southern Mali. Arid Lnd Res. Manag. 2004, 18, 359–368. [Google Scholar] [CrossRef]
- Prudencio, C.Y. Ring management of soils and crops in the west African semi-arid tropics: The case of the mossi farming system in Burkina Faso. Agric. Ecosyst. Environ. 1993, 47, 237–264. [Google Scholar] [CrossRef]
- Ramisch, J.J. Inequality, agro-pastoral exchanges, and soil fertility gradients in southern Mali. Agric. Ecosyst. Environ. 2005, 105, 353–372. [Google Scholar] [CrossRef]
- Wilding, L.P.; Smeck, N.E.; Hall, G.F. Pedogenesis and Soil Taxonomy. I. Concepts and Interactions; Elsevier Science: Amsterdam, The Netherlands, 1983; Chapter 4. [Google Scholar]
- Atzberger, C. Advances in remote sensing of agriculture: Context description, existing operational monitoring systems and major information needs. Remote Sens. 2013, 5, 949–981. [Google Scholar] [CrossRef]
- You, L.; Wood-Sichra, U.; Bacou, M.; Koo, J. Crop Production: SPAM. Available online: http://harvestchoice.org/tools/crop-production-spam-0 (accessed on 15 January 2016).
- STARS. Available online: http://www.stars-project.org/en/ (accessed on 7 December 2015).
- Blaes, X.; Traore, P.C.S.; Schut, A.G.T.; Ajeigbe, H.A.; Chomé, G.; Boekelo, B.; Diancoumba, M.; Goita, K.; Inuwa, A.H.; Zurita-Milla, R.; et al. STARS-ISABELA 2014–2015, Field Data Collection Protocol (Version 10, June 2015), project report. 2015; unpublished.
- Government of Mali/USAID. PIRT. Mali Land and Water Resources. Volumes II (Technical Report) and III (Appendices). Government of Mali/USAID/TAMS Ingénieurs; Tippetts-Abbett-McCarthy-Stratton: New York, NY, USA, 1983. [Google Scholar]
- Updike, T.; Comp, C. Radiometric Use Of Worldview-2 Imagery. Available online: http://global.digitalglobe.com/sites/default/files/Radiometric_Use_of_WorldView-2_Imagery%20(1).pdf (accessed on 21 June 2016).
- Podger, N.; Colwell, W.; Taylor, M. GeoEye-1 Radiance at Aperture and Planetary Reflectance. Available online: https://apollomapping.com/wp-content/user_uploads/2011/09/GeoEye1_Radiance_at_Aperture.pdf (accessed on 21 June 2016).
- Samake, A. Use of Locally Available Amendments to Improve Acid Soil Properties and Maize Yield in the Savanna Zone of Mali; Kwame Nkrumah University of Science and Technology: Kumasi, Ghana, 2014. [Google Scholar]
- Meier, U.; Biologische Bundesanstalt fur Land-und Forstwirtschaft. Growth stages of mono-and dicotyledonous plants BBCH Monograph. Agriculture 2001, 12, 14–27, 99–106. [Google Scholar]
- Weiss, M.; Baret, F. Can-Eye V6.313 User Manual. Available online: https://www6.paca.inra.fr/can-eye/Documentation-Publications/Documentation (accessed on 21 June 2016).
- Sensefly Sensefly Accessories. Available online: https://www.sensefly.com/drones/accessories.html (accessed on 20 June 2016).
- Tittonell, P.; Giller, K.E. When yield gaps are poverty traps: The paradigm of ecological intensification in African smallholder agriculture. Field Crops Res. 2013, 143, 76–90. [Google Scholar] [CrossRef]
- Tittonell, P.; Muriuki, A.; Klapwijk, C.J.; Shepherd, K.D.; Coe, R.; Vanlauwe, B. Soil heterogeneity and soil fertility gradients in smallholder farms of the east African highlands. Soil Sci. Soc. Am. J. 2013, 77, 525–538. [Google Scholar] [CrossRef]
- Chomé, G. Suivi des Cultures en Milieu Villageois Soudano-Sahélien par Télédétection à Très Hautes Résolutions: Analyse de la Détection des Niveaux de Fertilisation; Université Catholique de Louvain: Ottignies-Louvain-la-Neuve, Belgium, 2015. [Google Scholar]
- Van Evert, F.K.; Booij, R.; Jukema, J.N.; ten Berge, H.F.M.; Uenk, D.; Meurs, E.J.J.B.; van Geel, W.C.A.; Wijnholds, K.H.; Slabbekoorn, J.J.H. Using crop reflectance to determine sidedress N rate in potato saves N and maintains yield. Eur. J. Agron. 2012, 43, 58–67. [Google Scholar] [CrossRef]











| Acquisition Date | Satellite | Product Level | Product Type | DEM Correction | Correction Method |
|---|---|---|---|---|---|
| 1 May 2014 | GE01 | LV2A | Standard | Coarse DEM | 4 |
| 22 May 2014 | WV02 | LV3D | Orthorectified | Fine DEM | 4 |
| 30 May 2014 | WV02 | LV3D | Orthorectified | Fine DEM | 4 |
| 18 June 2014 | QB02 | LV2A | Ortho ready | Base Elevation | 2 |
| 24 June 2014 | GE01 | LV3D | Orthorectified | Fine DEM | 4 |
| 26 June 2014 | WV02 | LV2A | Ortho ready | Base Elevation | 2 |
| 8 July 2014 | GE01 | LV2A | Standard | Coarse DEM | 4 |
| 11 July 2014 | QB02 | LV2A | Ortho ready | Base Elevation | 2 |
| 29 July 2014 | WV02 | LV2A | Ortho ready | Base Elevation | 2 |
| 7 August 2014 | GE01 | LV3D | Orthorectified | Fine DEM | 4 |
| 26 August 2014 | GE01 | LV2A | Standard | Coarse DEM | 3 |
| 4 October 2014 | QB02 | LV2A | Standard | Coarse DEM | 3 |
| 18 October 2014 | WV02 | LV2A | Ortho ready | Base Elevation | 1 |
| 1 November 2014 | WV02 | LV2A | Ortho ready | Base Elevation | 1 |
| 14 November 2014 | WV02 | LV2A | Ortho ready | Base Elevation | 1 |
| Millet/Sorghum | DAP | 15-15-15 | Urea | Kg N/ha | Kg P/ha | Kg K/ha |
|---|---|---|---|---|---|---|
| B | 0.0 | 0.0 | 0.0 | |||
| C | 50 | 23.0 | 0.0 | 0.0 | ||
| D | 75 | 50 | 36.5 | 15.1 | 0.0 | |
| E | 150 | 50 | 50.0 | 30.1 | 0.0 | |
| F | 150 | 50 | 45.5 | 9.8 | 18.7 | |
| Maize | DAP | 15-15-15 | Urea | |||
| B | 100 | 150 | 84.0 | 6.5 | 12.5 | |
| C | 100 | 150 | 87.0 | 20.1 | 0.0 | |
| D | 200 | 300 | 168.0 | 13.1 | 24.9 | |
| E | 200 | 300 | 174.0 | 40.1 | 0.0 | |
| Peanut | DAP | |||||
| B | 0.0 | 0.0 | 0.0 | |||
| C | 50 | 9.0 | 10.0 | 0.0 | ||
| D | 100 | 18.0 | 20.1 | 0.0 | ||
| E | 150 | 27.0 | 30.1 | 0.0 | ||
| Cotton | Cotton Complex | Profeba | Urea | |||
| B | 0.0 | 0.0 | 0.0 | |||
| C | 150 | 50 | 44.0 | 11.8 | 22.4 | |
| D | 200 | 100 | 74.0 | 15.7 | 29.9 | |
| E | 200 | 10,000 | 100 | 109.6 | 43.1 | 6.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaes, X.; Chomé, G.; Lambert, M.-J.; Traoré, P.S.; Schut, A.G.T.; Defourny, P. Quantifying Fertilizer Application Response Variability with VHR Satellite NDVI Time Series in a Rainfed Smallholder Cropping System of Mali. Remote Sens. 2016, 8, 531. https://doi.org/10.3390/rs8060531
Blaes X, Chomé G, Lambert M-J, Traoré PS, Schut AGT, Defourny P. Quantifying Fertilizer Application Response Variability with VHR Satellite NDVI Time Series in a Rainfed Smallholder Cropping System of Mali. Remote Sensing. 2016; 8(6):531. https://doi.org/10.3390/rs8060531
Chicago/Turabian StyleBlaes, Xavier, Guillaume Chomé, Marie-Julie Lambert, Pierre Sibiry Traoré, Antonius G. T. Schut, and Pierre Defourny. 2016. "Quantifying Fertilizer Application Response Variability with VHR Satellite NDVI Time Series in a Rainfed Smallholder Cropping System of Mali" Remote Sensing 8, no. 6: 531. https://doi.org/10.3390/rs8060531
APA StyleBlaes, X., Chomé, G., Lambert, M. -J., Traoré, P. S., Schut, A. G. T., & Defourny, P. (2016). Quantifying Fertilizer Application Response Variability with VHR Satellite NDVI Time Series in a Rainfed Smallholder Cropping System of Mali. Remote Sensing, 8(6), 531. https://doi.org/10.3390/rs8060531