The Effect of Algal Blooms on Carbon Emissions in Western Lake Erie: An Integration of Remote Sensing and Eddy Covariance Measurements
Abstract
:1. Introduction
2. Materials and Methods
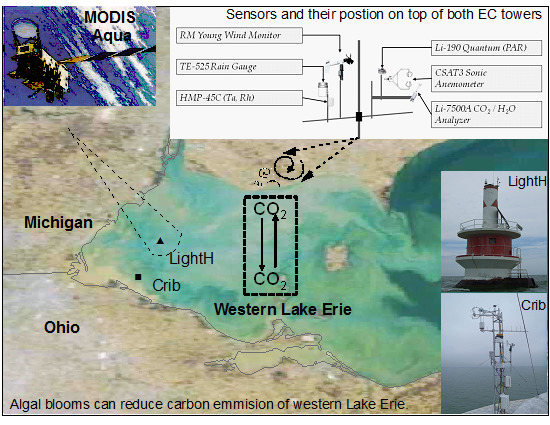
2.1. Study Area
2.2. Lake Carbon Cycling and Approaches
2.3. FCO2 and Meteorological Measurements
2.4. FCO2 Gap Filling
2.5. Field Measurement Chlorophyll-a
2.6. MODIS Chlorophyll-a Estimation
2.7. Statistical Analysis
3. Results
3.1. Algal Growth
3.2. Changes in FCO2
3.3. Meteorological Variables and FCO2
3.4. Chlorophyll-a and FCO2
4. Discussion
4.1. FCO2 and the Potential Drivers
4.2. Algal Blooms and Carbon Emissions
4.3. Limitations and Future Research
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanson, P.C.; Buffam, I.; Rusak, J.A.; Stanley, E.H.; Watras, C. Quantifying lake allochthonous organic carbon budgets using a simple equilibrium model. Limnol. Oceanogr. 2014, 59, 167–181. [Google Scholar] [CrossRef]
- Jonsson, A.; Algesten, G.; Bergstrom, A.K.; Bishop, K.; Sobek, S.; Tranvik, L.J.; Jansson, M. Integrating aquatic carbon fluxes in a boreal catchment carbon budget. J. Hydrol. 2007, 334, 141–150. [Google Scholar] [CrossRef]
- Jonsson, A.; Karlsson, J.; Jansson, M. Sources of carbon dioxide supersaturation in clearwater and humic lakes in Northern Sweden. Ecosystems 2003, 6, 224–235. [Google Scholar] [CrossRef]
- Buffam, I.; Turner, M.G.; Desai, A.R.; Hanson, P.C.; Rusak, J.A.; Lottig, N.R.; Stanley, E.H.; Carpenter, S.R. Integrating aquatic and terrestrial components to construct a complete carbon budget for a north temperate lake district. Glob. Chang. Biol. 2011, 17, 1193–1211. [Google Scholar] [CrossRef]
- Cole, J.J.; Caraco, N.F.; Kling, G.W.; Kratz, T.K. Carbon-dioxide supersaturation in the surface waters of lakes. Science 1994, 265, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef]
- Xiao, J.F.; Davis, K.J.; Urban, N.M.; Keller, K.; Saliendra, N.Z. Upscaling carbon fluxes from towers to the regional scale: Influence of parameter variability and land cover representation on regional flux estimates. J. Geophys. Res. Biogeosci. 2011. [Google Scholar] [CrossRef]
- Sun, G.; Caldwell, P.; Noormets, A.; McNulty, S.G.; Cohen, E.; Myers, J.M.; Domec, J.C.; Treasure, E.; Mu, Q.Z.; Xiao, J.F.; et al. Upscaling key ecosystem functions across the conterminous united states by a water centric ecosystem model. J. Geophys. Res. Biogeosci. 2011. [Google Scholar] [CrossRef]
- Jung, M.; Reichstein, M.; Margolis, H.A.; Cescatti, A.; Richardson, A.D.; Arain, M.A.; Arneth, A.; Bernhofer, C.; Bonal, D.; Chen, J.Q.; et al. Global patterns of land-atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. J. Geophys. Res. Biogeosci. 2011. [Google Scholar] [CrossRef]
- Hanson, P.C.; Pollard, A.I.; Bade, D.L.; Predick, K.; Carpenter, S.R.; Foley, J.A. A model of carbon evasion and sedimentation in temperate lakes. Glob. Change Biol. 2004, 10, 1285–1298. [Google Scholar] [CrossRef]
- Jonsson, A.; Aberg, J.; Lindroth, A.; Jansson, M. Gas transfer rate and CO2 flux between an unproductive lake and the atmosphere in Northern Sweden. J. Geophys. Res. Biogeosci. 2008. [Google Scholar] [CrossRef]
- Sobek, S.; Algesten, G.; BergstrÖM, A.K.; Jansson, M.; Tranvik, L.J. The catchment and climate regulation of pCO2 in boreal lakes. Glob. Change Biol. 2003, 9, 630–641. [Google Scholar] [CrossRef]
- Alin, S.R.; Johnson, T.C. Carbon cycling in large lakes of the world: A synthesis of production, burial, and lake-atmosphere exchange estimates. Glob. Biogeochem. Cycles 2007, 21, GB3002. [Google Scholar] [CrossRef]
- Finlay, K.; Leavitt, P.R.; Wissel, B.; Prairie, Y.T. Regulation of spatial and temporal variability of carbon flux in six hard-water lakes of the northern great plains. Limnol. Oceanogr. 2009, 54, 2553–2564. [Google Scholar] [CrossRef]
- Sellers, P.; Hesslein, R.H.; Kelly, C.A.K. Continuous measurement of CO2 for estimation of air-water fluxes in lakes: An in situ technique. Limnol. Oceanogr. 1995, 40, 575–581. [Google Scholar] [CrossRef]
- Eugster, W.; Kling, G.; Jonas, T.; McFadden, J.P.; Wuest, A.; MacIntyre, S.; Chapin, F.S. CO2 exchange between air and water in an Arctic Alaskan and midlatitude Swiss Lake: Importance of convective mixing. J. Geophys. Res. Atmos. 2003. [Google Scholar] [CrossRef]
- Bastviken, D.; Sundgren, I.; Natchimuthu, S.; Reyier, H.; Gålfalk, M. Technical note: Cost-efficient approaches to measure carbon dioxide (CO2) fluxes and concentrations in terrestrial and aquatic environments using mini loggers. Biogeosciences 2015, 12, 3849–3859. [Google Scholar] [CrossRef]
- Cole, J.J.; Caraco, N.F. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of sf6. Limnol. Oceanogr. 1998, 43, 647–656. [Google Scholar] [CrossRef]
- Morales-Pineda, M.; Cózar, A.; Laiz, I.; Úbeda, B.; Gálvez, J.Á. Daily, biweekly, and seasonal temporal scales of pCO2 variability in two stratified mediterranean reservoirs. J. Geophys. Res. Biogeosci. 2014, 119, 509–520. [Google Scholar] [CrossRef]
- Baldocchi, D.; Falge, E.; Wilson, K. A spectral analysis of biosphere-atmosphere trace gas flux densities and meteorological variables across hour to multi-year time scales. Agric. For. Meteorol. 2001, 107, 1–27. [Google Scholar] [CrossRef]
- Stoy, P.C.; Richardson, A.D.; Baldocchi, D.D.; Katul, G.G.; Stanovick, J.; Mahecha, M.D.; Reichstein, M.; Detto, M.; Law, B.E.; Wohlfahrt, G.; et al. Biosphere-atmosphere exchange of CO2 in relation to climate: A cross-biome analysis across multiple time scales. Biogeosciences 2009, 6, 2297–2312. [Google Scholar] [CrossRef]
- Yi, C.X.; Ricciuto, D.; Li, R.; Wolbeck, J.; Xu, X.Y.; Nilsson, M.; Aires, L.; Albertson, J.D.; Ammann, C.; Arain, M.A.; et al. Climate control of terrestrial carbon exchange across biomes and continents. Environ. Res. Lett. 2010. [Google Scholar] [CrossRef]
- Anderson, D.E.; Striegl, R.G.; Stannard, D.I.; Michmerhuizen, C.M.; McConnaughey, T.A.; LaBaugh, J.W. Estimating lake-atmosphere CO2 exchange. Limnol. Oceanogr. 1999, 44, 988–1001. [Google Scholar] [CrossRef]
- Vesala, T.; Huotari, J.; Rannik, U.; Suni, T.; Smolander, S.; Sogachev, A.; Launiainen, S.; Ojala, A. Eddy covariance measurements of carbon exchange and latent and sensible heat fluxes over a boreal lake for a full open-water period. J. Geophys. Res. Atmos. 2006. [Google Scholar] [CrossRef]
- Huotari, J.; Ojala, A.; Peltomaa, E.; Nordbo, A.; Launiainen, S.; Pumpanen, J.; Rasilo, T.; Hari, P.; Vesala, T. Long-term direct CO2 flux measurements over a boreal lake: Five years of eddy covariance data. Geophys. Res. Lett. 2011. [Google Scholar] [CrossRef]
- Anderson, D.; Glibert, P.; Burkholder, J. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Davies, W.; Nugegoda, D. Harmful algal blooms in the Gippsland Lakes, Victoria: A review. Proce. R. Soc. Vic. 2012, 124, 179–192. [Google Scholar]
- Steffen, M.M.; Belisle, B.S.; Watson, S.B.; Boyer, G.L.; Wilhelm, S.W. Status, causes and controls of cyanobacterial blooms in Lake Erie. J. Gt. Lakes Res. 2014, 40, 215–225. [Google Scholar] [CrossRef]
- Commission, I.J. Phosphorus Management for the Great Lakes; International Joint Commission, Great Lakes Science Advisory Board, Great Lakes Water Quality Board: Windsor, ON, Canada, 1980. [Google Scholar]
- Bridgeman, T.B.; Chaffin, J.D.; Filbrun, J.E. A novel method for tracking Western Lake Erie microcystis blooms, 2002–2011. J. Gt. Lakes Res. 2013, 39, 83–89. [Google Scholar] [CrossRef]
- Bukata, R.P. Satellite Monitoring of Inland and Coastal Water Quality; CRC: New York, NY, USA, 2005. [Google Scholar]
- O'Reilly, J.E. Ocean Color Chlorophyll a Algorithms for Seawifs, OC2 and OC4: Version 4; NOAA, National Marine Fisheries Service: Narragansett, RI, USA, 2000. [Google Scholar]
- Xiao, J.F.; Sun, G.; Chen, J.Q.; Chen, H.; Chen, S.P.; Dong, G.; Gao, S.H.; Guo, H.Q.; Guo, J.X.; Han, S.J.; et al. Carbon fluxes, evapotranspiration, and water use efficiency of terrestrial ecosystems in China. Agric. For. Meteorol. 2013, 182, 76–90. [Google Scholar] [CrossRef]
- Herdendorf, C.E.; Monaco, M.E. Physical and Chemical Limnology of the Island Region of Lake Erie; Ohio State University: Columbus, OH, USA, 1988. [Google Scholar]
- Paul, J.F.; Kasprzyk, R.; Lick, W. Turbidity in the western basin of Lake Erie. J. Geophys. Res. Ocean. 1982, 87, 5779–5784. [Google Scholar] [CrossRef]
- Bertram, P.E. Total phosphorus and dissolved-oxygen trends in the central basin of Lake Erie, 1970–1991. J. Gt. Lakes Res. 1993, 19, 224–236. [Google Scholar] [CrossRef]
- Shao, C.; Chen, J.; Stepien, C.A.; Chu, H.; Ouyang, Z.; Bridgeman, T.B.; Czajkowski, K.P.; Becker, R.H.; John, R. Diurnal to annual changes in latent, sensible heat, and CO2 fluxes over a Laurentian Great Lake: A case study in western Lake Erie. J. Geophys. Res. Biogeosci. 2015, 120, 1587–1604. [Google Scholar] [CrossRef]
- Wilczak, J.M.; Oncley, S.P.; Stage, S.A. Sonic anemometer tilt correction algorithms. Bound. Layer Meteorol. 2001, 99, 127–150. [Google Scholar] [CrossRef]
- Schotanus, P.; Nieuwstadt, F.T.M.; Bruin, H.A.R. Temperature measurement with a sonic anemometer and its application to heat and moisture fluxes. Bound. Layer Meteorol. 1983, 26, 81–93. [Google Scholar] [CrossRef]
- Massman, W.J.; Lee, X. Eddy covariance flux corrections and uncertainties in long-term studies of carbon and energy exchanges. Agric. For. Meteorol. 2002, 113, 121–144. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water-vapor transfer. Quart. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Grelle, A.; Burba, G. Fine-wire thermometer to correct CO2 fluxes by open-path analyzers for artificial density fluctuations. Agric. For. Meteorol. 2007, 147, 48–57. [Google Scholar] [CrossRef]
- Foken, T.; Wichura, B. Tools for quality assessment of surface-based flux measurements. Agric. For. Meteorol. 1996, 78, 83–105. [Google Scholar] [CrossRef]
- Chu, H.; Gottgens, J.F.; Chen, J.; Sun, G.; Desai, A.R.; Ouyang, Z.; Shao, C.; Czajkowski, K. Climatic variability, hydrologic anomaly, and methane emission can turn productive freshwater marshes into net carbon sources. Glob. Change Biol. 2014, 3, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chen, J.; Gottgens, J.F.; Ouyang, Z.; John, R.; Czajkowski, K.; Becker, R. Net ecosystem methane and carbon dioxide exchanges in a lake erie coastal marsh and a nearby cropland. J. Geophys. Res. Biogeosci. 2014. [Google Scholar] [CrossRef]
- Ouyang, Z.; Chen, J.; Becker, R.; Chu, H.; Xie, J.; Shao, C.; John, R. Disentangling the confounding effects of PAR and air temperature on net ecosystem exchange at multiple time scales. Ecol. Complex. 2014, 19, 46–58. [Google Scholar] [CrossRef]
- Ikawa, H.; Faloona, I.; Kochendorfer, J.; Paw U, K.T.; Oechel, W.C. Air–sea exchange of CO2 at a Northern California coastal site along the california current upwelling system. Biogeosciences 2013, 10, 4419–4432. [Google Scholar] [CrossRef]
- Podgrajsek, E.; Sahlee, E.; Bastviken, D.; Holst, J.; Lindroth, A.; Tranvik, L.; Rutgersson, A. Comparison of floating chamber and eddy covariance measurements of lake greenhouse gas fluxes. Biogeosciences 2014, 11, 4225–4233. [Google Scholar] [CrossRef] [Green Version]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Bridgeman, T.B.; Morris, J. Changes in water quality of Maumee Bay, Lake Erie: 1974–2002. In Checking the Pulse of Lake Erie; Munawar, M., Heath, R., Eds.; Michigan State University Press: East Lansing, MI, USA, 2008; pp. 129–130. [Google Scholar]
- Lesht, B.M.; Barbiero, R.P.; Warren, G.J. Satellite ocean color algorithms: A review of applications to the Great Lakes. J. Gt. Lakes Res. 2012, 38, 49–60. [Google Scholar] [CrossRef]
- Maritorena, S.; Siegel, D.A.; Peterson, A.R. Optimization of a semianalytical ocean color model for global-scale applications. Appl. Opt. 2002, 41, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Franz, B.A.; Werdell, P.J. A generalized framework for modeling of inherent optical properties in remote sensing applications. In Proceedings of the 2010 Ocean Optics XX Conference, Anchorage, AL, USA, 25 September–1 October 2010.
- Franz, B.A.; Werdell, P.J.; Meister, G.; Kwiatkowska, E.J.; Bailey, S.W.; Ahmad, Z.; McClain, C.R. MODIS land bands for ocean remote sensing applications. In Proceedings of the 2006 Ocean Optics XVIII Conference, Montreal, QB, Canada, 9–13 October 2006.
- The Ocean Color Web. Available online: https://oceancolor.gsfc.nasa.gov/cms/ (accessed on 12 December 2016).
- Campbell, J.W.; Blaisdell, J.M.; Darzi, M. Level-3 Seawifs Data Products: SPATIAL and Temporal Binning AlgorIthms; NASA: Greenbelt, MD, USA, 1995; Volume 32.
- Ho, J.C.; Michalak, A.M. Challenges in tracking harmful algal blooms: A synthesis of evidence from Lake Erie. J. Gt. Lakes Res. 2015, 41, 317–325. [Google Scholar] [CrossRef]
- Solomon, C.T.; Bruesewitz, D.A.; Richardson, D.C.; Rose, K.C.; Van de Bogert, M.C.; Hanson, P.C.; Kratz, T.K.; Larget, B.; Adrian, R.; Babin, B.L.; et al. Ecosystem respiration: Drivers of daily variability and background respiration in lakes around the globe. Limnol. Oceanogr. 2013, 58, 849–866. [Google Scholar] [CrossRef]
- Coloso, J.J.; Cole, J.J.; Pace, M.L. Difficulty in discerning drivers of lake ecosystem metabolism with high-frequency data. Ecosystems 2011, 14, 935–948. [Google Scholar] [CrossRef]
- Dodson, S.I. Introduction to Limnology; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Balmer, M.B.; Downing, J.A. Carbon dioxide concentrations in eutrophic lakes: Undersaturation implies atmospheric uptake. Inland Waters 2011, 1, 125–132. [Google Scholar] [CrossRef]
- Lennon, J.T. Experimental evidence that terrestrial carbon subsidies increase CO2 flux from lake ecosystems. Oecologia 2004, 138, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Hollinger, D.Y.; Burba, G.G.; Davis, K.J.; Flanagan, L.B.; Katul, G.G.; Munger, J.W.; Ricciuto, D.M.; Stoy, P.C.; Suyker, A.E.; et al. A multi-site analysis of random error in tower-based measurements of carbon and energy fluxes. Agric. For. Meteorol. 2006, 136, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Verdú, A.; Jiménez, J.C.; Lazzaro, X.; Tenjo, C.; Delegido, J.; Pereira, M.; Sobrino, J.A.; Moreno, J. Comparison of MODIS and Landsat-8 retrievals of chlorophyll-a and water temperature over Lake Titicaca. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–15 July 2016; pp. 7643–7646.
- Tobias, C.R.; Böhlke, J.K.; Harvey, J.W. The oxygen-18 isotope approach for measuring aquatic metabolism in high productivity waters. Limnol. Oceanogr. 2007, 52, 1439–1453. [Google Scholar] [CrossRef]








| Site | Year | U (m·s−1) | Ta (°C) | PAR (mol·m−2·day−1) |
|---|---|---|---|---|
| LightH | 2012 | 5.55 ± 2.20 | 16.6 ± 7.5 | 32.64 ± 15.2 |
| 2013 | 6.37 ± 2.22 | 15.9 ± 7.6 | 30.72 ± 13.9 | |
| 2014 | 6.39 ± 2.27 | 15.9 ± 6.2 | 29.91 ± 13.8 | |
| Crib | 2012 | 4.93 ± 2.04 | 16.6 ± 7.4 | 32.64 ± 15.2 |
| 2013 | 5.50 ± 2.10 | 15.4 ± 7.4 | 30.72 ± 13.9 | |
| 2014 | 5.36 ± 2.21 | 15.3 ± 6.2 | 29.91 ± 13.8 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Z.; Shao, C.; Chu, H.; Becker, R.; Bridgeman, T.; Stepien, C.A.; John, R.; Chen, J. The Effect of Algal Blooms on Carbon Emissions in Western Lake Erie: An Integration of Remote Sensing and Eddy Covariance Measurements. Remote Sens. 2017, 9, 44. https://doi.org/10.3390/rs9010044
Ouyang Z, Shao C, Chu H, Becker R, Bridgeman T, Stepien CA, John R, Chen J. The Effect of Algal Blooms on Carbon Emissions in Western Lake Erie: An Integration of Remote Sensing and Eddy Covariance Measurements. Remote Sensing. 2017; 9(1):44. https://doi.org/10.3390/rs9010044
Chicago/Turabian StyleOuyang, Zutao, Changliang Shao, Housen Chu, Richard Becker, Thomas Bridgeman, Carol A. Stepien, Ranjeet John, and Jiquan Chen. 2017. "The Effect of Algal Blooms on Carbon Emissions in Western Lake Erie: An Integration of Remote Sensing and Eddy Covariance Measurements" Remote Sensing 9, no. 1: 44. https://doi.org/10.3390/rs9010044
APA StyleOuyang, Z., Shao, C., Chu, H., Becker, R., Bridgeman, T., Stepien, C. A., John, R., & Chen, J. (2017). The Effect of Algal Blooms on Carbon Emissions in Western Lake Erie: An Integration of Remote Sensing and Eddy Covariance Measurements. Remote Sensing, 9(1), 44. https://doi.org/10.3390/rs9010044