Using Worldview Satellite Imagery to Map Yield in Avocado (Persea americana): A Case Study in Bundaberg, Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Crop Status
2.2. Satellite Imagery and Pre-Processing
2.3. Sampling Trees
2.4. Extraction of Spectral Data
2.5. Selection of VIs and Data Analysis
2.6. Derivation of Block Level Yield Maps and Predictions of Average Block Yield
3. Results
3.1. VIs for Estimatin of Yield Parameters
3.2. Models for Relationship between Yield Parameters and VIs
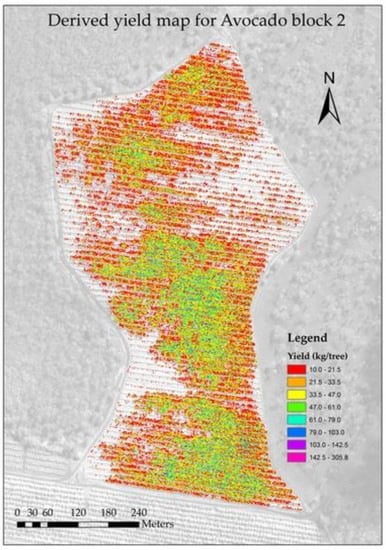
3.3. Accuracy of Block Level Yield Maps
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Papageorgiou, E.I.; Aggelopoulou, K.D.; Gemtos, T.A.; Nanos, G.D. Yield prediction in apples using Fuzzy Cognitive Map learning approach. Comput. Electron. Agric. 2013, 91, 19–29. [Google Scholar] [CrossRef]
- Wang, Q.; Nuske, S.; Bergerman, M.; Singh, S. Automated Crop Yield Estimation for Apple Orchards. In Experimental Robotics: The 13th International Symposium on Experimental Robotics; Desai, P.J., Dudek, G., Khatib, O., Kumar, V., Eds.; Springer International Publishing: Heidelberg, Germany, 2013; pp. 745–758. [Google Scholar]
- Köhne, S. Yield estimation based on measureable parameters. S. Afr. Avocado Grow. Assoc. Yearb. 1985, 8, 103:1–103:3. [Google Scholar]
- Robson, A.J.; Petty, J.; Joyce, D.C.; Marques, J.R.; Hofman, P.J. High resolution remote sensing, GIS and Google Earth for avocado fruit quality mapping and tree number auditing. In Proceedings of the 29th International Horticultural Congress, Brisbane Convention and Exhibition Centre, Brisbane, Australia, 17–22 August 2014; pp. 589–595. [Google Scholar]
- Zamora, F.A.; Téllez, C.P.; Wulfsohn, D.; Zamora, I.; García-Fiñana, M. Performance of a procedure for fruit yield estimation. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, Pittsburgh, PA, USA, 20–23 June 2010; pp. 5275–5290. [Google Scholar]
- Winter, F. A Simulation Model for Studying the Efficiency of Apple and Pear Orchards/Ein Simulationsmodell zur Untersuchung der Wirtschaftlichkeit von Apfel-und Birnenanlagen. Gartenbauwissenschaft 1976, 14, 26–34. [Google Scholar]
- Aggelopoulou, K.D.; Wulfsohn, D.; Fountas, S.; Gemtos, T.A.; Nanos, G.D.; Blackmore, S. Spatial variation in yield and quality in a small apple orchard. Precis. Agric. 2010, 11, 538–556. [Google Scholar] [CrossRef]
- Alburquerque, N.; Burgos, L.; Egea, J. Influence of flower bud density, flower bud drop and fruit set on apricot productivity. Sci. Hortic. 2004, 102, 397–406. [Google Scholar] [CrossRef]
- Whiley, A.W.; Saranah, J.B.; Rasmussen, T.S. The Relationship between Carbohydrate Levels and Productivity in the Avocado and Impact on Management Practices, Particularly Time of Harvest; Horticultural Research and Development Corporation: Gordon, Australia, 1996.
- Zaman, Q.; Schumann, A.; Percival, D.; Gordon, R. Estimation of wild blueberry fruit yield using digital color photography. Trans. ASABE 2008, 51, 1539–1544. [Google Scholar] [CrossRef]
- Zhou, R.; Damerow, L.; Sun, Y.; Blanke, M.M. Using colour features of cv. ‘Gala’ apple fruits in an orchard in image processing to predict yield. Precis. Agric. 2012, 13, 568–580. [Google Scholar] [CrossRef]
- Annamalai, P.; Lee, W.S.; Burks, T. Color Vision Systems for Estimating Citrus Yield in Real-Time; ASAE Paper No. 040354; ASAE: St. Joseph, MI, USA, 2004. [Google Scholar]
- Bulanon, D.M.; Kataoka, T.; Ota, Y.; Hiroma, T. AE—Automation and Emerging Technologies. Biosyst. Eng. 2002, 83, 405–412. [Google Scholar] [CrossRef]
- Lee, W.S. Citrus Yield Mapping System in Natural Outdoor Scenes using the Watershed Transform. In Proceedings of the 2006 ASABE Annual International Meeting Sponsored, ASABE Oregon Convention Center, Portland, OR, USA, 9–12 July 2006. [Google Scholar]
- Stajnko, D.; Lakota, M.; Hočevar, M. Estimation of number and diameter of apple fruits in an orchard during the growing season by thermal imaging. Comput. Electron. Agric. 2004, 42, 31–42. [Google Scholar] [CrossRef]
- Doraiswamy, P.C.; Moulin, S.; Cook, P.W.; Stern, A. Crop yield assessment from remote sensing. Photogramm. Eng. Remote Sens. 2003, 69, 665–674. [Google Scholar] [CrossRef]
- Prasad, A.K.; Chai, L.; Singh, R.P.; Kafatos, M. Crop yield estimation model for Iowa using remote sensing and surface parameters. Int. J. Appl. Earth Obs. Geoinf. 2006, 8, 26–33. [Google Scholar] [CrossRef]
- Shanahan, J.F.; Schepers, J.S.; Francis, D.D.; Varvel, G.E.; Wilhelm, W.W.; Tringe, J.M.; Schlemmer, M.R.; Major, D.J. Use of Remote-Sensing Imagery to Estimate Corn Grain Yield. Agron. J. 2001, 93, 583–589. [Google Scholar] [CrossRef]
- Rahman, M.M.; Robson, A.J. A Novel Approach for Sugarcane Yield Prediction Using Landsat Time Series Imagery: A Case Study on Bundaberg Region. Adv. Remote Sens. 2016, 5, 93–102. [Google Scholar] [CrossRef]
- Robson, A.J. Remote Sensing Applications for the Determination of Yield, Maturity and Aflatoxin Contamination in Peanut. Ph.D. Thesis, University of Queensland, St. Lucia, Australia, 2007. [Google Scholar]
- Rembold, F.; Atzberger, C.; Savin, I.; Rojas, O. Using Low Resolution Satellite Imagery for Yield Prediction and Yield Anomaly Detection. Remote Sens. 2013, 5, 1704–1733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, S.; Sun, H.; Pei, D.; Wang, Y. Dry matter, harvest index, grain yield and water use efficiency as affected by water supply in winter wheat. Irrig. Sci. 2008, 27, 1–10. [Google Scholar] [CrossRef]
- Cannell, M.G.R. Dry matter partitioning in tree crops. In Attributes of Trees as Crop Plants; Cannell, M.G.R., Jackson, J.E., Eds.; Institute of Terrestrial Ecology: Abbotts Ripton, UK, 1985; pp. 160–193. [Google Scholar]
- Somers, B.; Delalieux, S.; Verstraeten, W.; Vanden Eynde, A.; Barry, G.; Coppin, P. The contribution of the fruit component to the hyperspectral citrus canopy signal. Photogramm. Eng. Remote Sens. 2010, 76, 37–47. [Google Scholar] [CrossRef]
- Ye, X.; Sakai, K.; Manago, M.; Asada, S.-I.; Sasao, A. Prediction of citrus yield from airborne hyperspectral imagery. Precis. Agric. 2007, 8, 111–125. [Google Scholar] [CrossRef]
- Best, S.; Salazar, F.; Bastias, R.; Leon, L. Crop-load estimation model to optimize yield-Quality ratio in apple orchards, Malus Domestic Borkh, var. Royal Gala. J. Inf. Technol. Agric. 2008, 3, 11–18. [Google Scholar]
- Van Beek, J.; Tits, L.; Somers, B.; Deckers, T.; Verjans, W.; Bylemans, D.; Janssens, P.; Coppin, P. Temporal Dependency of Yield and Quality Estimation through Spectral Vegetation Indices in Pear Orchards. Remote Sens. 2015, 7, 9886–9903. [Google Scholar] [CrossRef]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.J.; Jiménez-Muñoz, J.C.; Sobrino, J.A.; Soriano, M.A.; Fereres, E.; Vega, V.; Pastor, M. Monitoring yield and fruit quality parameters in open-canopy tree crops under water stress. Implications for ASTER. Remote Sens. Environ. 2007, 107, 455–470. [Google Scholar] [CrossRef]
- Payne, A.B.; Walsh, K.B.; Subedi, P.P.; Jarvis, D. Estimation of mango crop yield using image analysis—Segmentation method. Comput. Electron. Agric. 2013, 91, 57–64. [Google Scholar] [CrossRef]
- Serrano, L.; González-Flor, C.; Gorchs, G. Assessment of grape yield and composition using the reflectance based Water Index in Mediterranean rainfed vineyards. Remote Sens. Environ. 2012, 118, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.; Lamb, D.W.; Holzapfel, B.; Louis, J. Optical remote sensing applications in viticulture—A review. Aust. J. Grape Wine Res. 2002, 8, 36–47. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resources Perspective; Prentice-Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium—Volume I: Technical Presentations; NASA SP-351; NASA: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Formulation and assessment of narrow-band vegetation indices from EO-1 Hyperion imagery for discriminating sugarcane disease. In Proceedings of the Spatial Sciences Institute Biennial Conference (SSC 2003): Spatial Knowledge Without Boundaries, Canberra, Australia, 22–26 September 2003. [Google Scholar]
- Maitra, S.; Yan, J. Principle component analysis and partial least squares: Two dimension reduction techniques for regression. Appl. Multivar. Stat. Models 2008, 79, 79–90. [Google Scholar]
- Tobias, R.D. An introduction to partial least squares regression. In Proceedings of the Twentieth Annual SAS Users Group International Conference; SAS: Cary, NC, USA, 1995; pp. 1250–1257. [Google Scholar]
- BOM. Bureau of Meteorology. Available online: http://www.bom.gov.au (accessed on 7 July 2017).
- Newett, S.; Whiley, A.; Dirou, J.; Hofman, P.; Ireland, G.; Kernot, L.; Ledger, S.; McCarthy, A.; Miller, J.; Pinese, B.; et al. Avocado Information Kit; Agrilink Series QAL9906; Department of Primary Industries: Taabinga, Australia, 2001.
- Whiley, A.; Hargreaves, P.; Pegg, K.; Doogan, V.; Ruddle, L.; Saranah, J.; Langdon, P. Changing sink strengths influence translocation of phosphonate in avocado (Persea americana Mill.) trees. Aust. J. Agric. Res. 1995, 46, 1079–1090. [Google Scholar] [CrossRef]
- Kuester, M. Radiometric Use of WorldView-3 Imagery; DigitalGlobe: Longmont, CO, USA, 2016. [Google Scholar]
- Rueda, C.A.; Greenberg, J.A.; Ustin, S.L. StarSpan: A Tool for Fast Selective Pixel Extraction from Remotely Sensed Data; Center for Spatial Technologies and Remote Sensing (CSTARS), University of California at Davis: Davis, CA, USA, 2005. [Google Scholar]
- Gobron, N.; Pinty, B.; Verstraete, M.M.; Widlowski, J.-L. Advanced vegetation indices optimized for up-coming sensors: Design, performance, and applications. IEEE Trans. Geosci. Remote Sens. 2000, 38, 2489–2505. [Google Scholar]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of Red Edge Position and Chlorophyll Content by Reflectance Measurements Near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Pu, R.; Landry, S. A comparative analysis of high spatial resolution IKONOS and WorldView-2 imagery for mapping urban tree species. Remote Sens. Environ. 2012, 124, 516–533. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Bannari, A.; Asalhi, H.; Teillet, P.M. Transformed difference vegetation index (TDVI) for vegetation cover mapping. In Proceedings of the 2002 IEEE International Conference on Geoscience and Remote Sensing Symposium, Toronto, ON, Canada, 24–28 June 2002; Volume 3055, pp. 3053–3055. [Google Scholar]
- Goel, N.S.; Qin, W. Influences of canopy architecture on relationships between various vegetation indices and LAI and FPAR: A computer simulation. Remote Sens. Rev. 1994, 10, 309–347. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing (Version 3.3.3); R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Lötze, E.; Bergh, O. Early prediction of harvest fruit size distribution of an apple and pear cultivar. Sci. Hortic. 2004, 101, 281–290. [Google Scholar] [CrossRef]
- Whiley, A.W.; Saranah, J.B.; Wolstenholme, B.N. Pheno-physiological modelling in avocado—An aid in research planning. In Proceedings of the World Avocado Congress III, Tel Aviv, Israel, 22–27 October 1995; pp. 71–75. [Google Scholar]
- Curran, P.J.; Windham, W.R.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 1995, 15, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, B.A.; Wolstenholme, B.N.; Whiley, A.W. The Avocado: Botany, Production and Uses; CABI: Wallingford, UK, 2013. [Google Scholar]










| NAME | FORMULA | REFERENCES | |
|---|---|---|---|
| 1 | Normalized Difference Rededge/Red (NDVI rededge) | (RE − R)/(RE + R) | [43] |
| 2 | Transformed Chlorophyll Absorption in Reflectance Index (TCARI) | 3 × ((RE − R) − 0.2 × (RE − G) × (RE/R)) | [44] |
| 3 | Structure Insensitive Pigment Index (SIPI) | (NIR1 − B)/(NIR1 + R) | [45] |
| 4 | Coastal Blue Structure Insensitive Pigment Index (CB SIPI) | (NIR1 − CB)/(NIR1 + CB) | [45] |
| 5 | Normalized difference Red/Red-edge index (R/RENDVI) | (NIR1 − R)/(NIR1 + RE) | [46] |
| 6 | Normalized difference Red/NIR2 index (R/N2NDVI) | (NIR1 − R)/(NIR1 + NIR2) | [4] |
| 7 | Green normalized difference vegetation index (GNDVI) | (NIR1 − G)/(NIR1 + G) | [47] |
| 8 | Modified Simple Ratio (MSR) | (NIR1/R − 1)/(SQRT((NIR1/R) + 1)) | [48] |
| 9 | Ratio Vegetation Index (RVI) | NIR1/R | [34] |
| 10 | Normalized Difference Vegetation Index (N1NDVI) | (NIR1 − R)/(NIR1 + R) | [33] |
| 11 | Normalized Difference Vegetation Index (N2NDVI) | (NIR2 − R)/(NIR2 + R) | [33] |
| 12 | Normalized difference red edge index 1 (RENDVI1) | (NIR1 − RE)/(NIR1 + RE) | [49] |
| 13 | Normalized difference red edge index 2 (RENDVI2) | (NIR2 − RE)/(NIR2 + RE) | [50] |
| 14 | RDVI1 | (NIR1 − R)/(SQRT (NIR1 + R)) | [51] |
| 15 | RDVI2 | (NIR2 − R)/(SQRT (NIR2 + R)) | [51] |
| 16 | Transformed difference vegetation index (TDVI) | 1.5 × ((NIR1 − R)/(SQRT(NIR12 + R + 0.5)) | [52] |
| 17 | Transformed difference vegetation index 2 (TDVI2) | 1.5 × ((NIR2 − R)/(SQRT(NIR22 + R + 0.5)) | [52] |
| 18 | Non Linear Index (NLI) | (NIR2 − R)/(NIR2 + R) | [53] |
| Blocks | Total Fruit Weight (kg·tree−1) | Average Fruit Size (g) Per Tree | ||||
|---|---|---|---|---|---|---|
| PCA | Non-Linear Regression | R2 | PCA | Non-Linear Regression | R2 | |
| All blocks for 3 years | RENDVI1, RENDVI2 | RENDVI1 | 0.29 | RENDVI1, RENDVI2 | RENDVI1 | 0.29 |
| block 1 (3 years) | RENDVI1, N1GNDVI | N1GNDVI | 0.48 | RENDVI1, N1/RENDVI | RENDVI1 | 0.39 |
| Block 2 (3 years) | RENDVI1, RENDVI2 | RENDVI2 | 0.25 | RENDVI1, RENDVI2 | RENDVI1 | 0.43 |
| Block 3 (2 years) | CB SIPI, Yellow SAVI | CB SIPI | 0.40 | RENDVI2, CB SIPI | RENDVI2 | 0.28 |
| Block 4 (2 years) | SIPI, Yellow SAVI | SIPI | 0.43 | Yellow SAVI, RDVI | Yellow SAVI | 0.19 |
| Block 5 (2 years) | RENDVI2, RENDVI1 | RENDVI2 | 0.49 | RENDVI2, RENDVI1 | RENDVI2 | 0.40 |
| Blocks | Slopes | Intercepts | ||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| Block 1 | 0.72 | 0.49 | 16 | 0 |
| Block 2 | 2.85 | 0.07 | 11.6 | 0.0001 |
| Block 3 | 0.23 | 0.63 | 4.72 | 0.04 |
| Block 4 | 0.23 | 0.63 | 3.98 | 0.05 |
| Block 5 | 2.87 | 0.10 | 0.67 | 0.42 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robson, A.; Rahman, M.M.; Muir, J. Using Worldview Satellite Imagery to Map Yield in Avocado (Persea americana): A Case Study in Bundaberg, Australia. Remote Sens. 2017, 9, 1223. https://doi.org/10.3390/rs9121223
Robson A, Rahman MM, Muir J. Using Worldview Satellite Imagery to Map Yield in Avocado (Persea americana): A Case Study in Bundaberg, Australia. Remote Sensing. 2017; 9(12):1223. https://doi.org/10.3390/rs9121223
Chicago/Turabian StyleRobson, Andrew, Muhammad Moshiur Rahman, and Jasmine Muir. 2017. "Using Worldview Satellite Imagery to Map Yield in Avocado (Persea americana): A Case Study in Bundaberg, Australia" Remote Sensing 9, no. 12: 1223. https://doi.org/10.3390/rs9121223
APA StyleRobson, A., Rahman, M. M., & Muir, J. (2017). Using Worldview Satellite Imagery to Map Yield in Avocado (Persea americana): A Case Study in Bundaberg, Australia. Remote Sensing, 9(12), 1223. https://doi.org/10.3390/rs9121223