Spectroscopic Estimation of Biomass in Canopy Components of Paddy Rice Using Dry Matter and Chlorophyll Indices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Spectral Measurements
2.3. Biomass Measurements
2.4. Calculation of Spectral Indices and Estimation of Biomass
3. Results
3.1. Variation in Biomass of Individual and Multiple Components over the Growing Season
3.2. Relationships between Vegetation Indices and Biomass of Individual Components
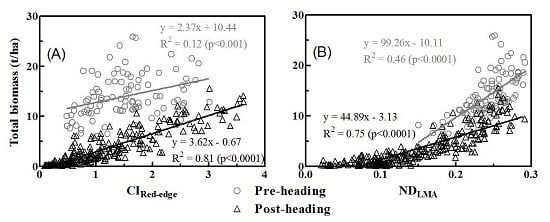
3.3. Relationships between Vegetation Indices and Biomass of Multiple Components
3.4. Model Validation
4. Discussion
4.1. Why Did Dry Matter Indices Work Better Than Chlorophyll Indices?
4.2. Partitioning of Aboveground Biomass between Canopy Components
4.3. Potential for Satellite Observations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cantrell, R.P.; Reeves, T.G. The cereal of the world’s poor takes center stage. Science 2002, 296, 53–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Miao, Y.; Wang, H.; Su, M.; Fan, M.; Zhang, F.; Jiang, R.; Zhang, Z.; Liu, C.; Liu, P. A preliminary precision rice management system for increasing both grain yield and nitrogen use efficiency. Field Crop. Res. 2013, 154, 23–30. [Google Scholar] [CrossRef]
- Harrell, D.; Tubana, B.; Walker, T.; Phillips, S. Estimating rice grain yield potential using normalized difference vegetation index. Agron. J. 2011, 103, 1717–1723. [Google Scholar] [CrossRef]
- Peng, Y.; Gitelson, A.A. Application of chlorophyll-related vegetation indices for remote estimation of maize productivity. Agric. For. Meteorol. 2011, 151, 1267–1276. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Casanova, D.; Epema, G.F.; Goudriaan, J. Monitoring rice reflectance at field level for estimating biomass and lai. Field Crop Res. 1998, 55, 83–92. [Google Scholar] [CrossRef]
- Serrano, L.; Filella, I.; Penuelas, J. Remote sensing of biomass and yield of winter wheat under different nitrogen supplies. Crop Sci. 2000, 40, 723–731. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Yang, G.J.; Wang, J.H.; Song, X.Y.; Feng, H.K. Winter wheat biomass estimation based on spectral indices, band depth analysis and partial least squares regression using hyperspectral measurements. Comput. Electron. Agric. 2014, 100, 51–59. [Google Scholar] [CrossRef]
- Dong, T.F.; Liu, J.G.; Qian, B.D.; Jing, Q.; Croft, H.; Chen, J.M.; Wang, J.F.; Huffman, T.; Shang, J.L.; Chen, P.F. Deriving maximum light use efficiency from crop growth model and satellite data to improve crop biomass estimation. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 104–117. [Google Scholar] [CrossRef]
- He, B.B.; Li, X.; Quan, X.W.; Qiu, S. Estimating the aboveground dry biomass of grass by assimilation of retrieved lai into a crop growth model. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 550–561. [Google Scholar] [CrossRef]
- Jego, G.; Pattey, E.; Liu, J.G. Using leaf area index, retrieved from optical imagery, in the stics crop model for predicting yield and biomass of field crops. Field Crop. Res. 2012, 131, 63–74. [Google Scholar] [CrossRef]
- Liu, J.G.; Pattey, E.; Miller, J.R.; McNairn, H.; Smith, A.; Hu, B.X. Estimating crop stresses, aboveground dry biomass and yield of corn using multi-temporal optical data combined with a radiation use efficiency model. Remote Sens. Environ. 2010, 114, 1167–1177. [Google Scholar] [CrossRef]
- Jin, X.L.; Yang, G.J.; Xu, X.G.; Yang, H.; Feng, H.K.; Li, Z.H.; Shen, J.X.; Zhao, C.J.; Lan, Y.B. Combined multi-temporal optical and radar parameters for estimating lai and biomass in winter wheat using HJ and RadarSAT-2 data. Remote Sens. 2015, 7, 13251–13272. [Google Scholar] [CrossRef]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating biomass of barley using Crop Surface Models (CSMs) derived from UAV-based RGB imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Magney, T.S.; Vierling, L.A.; Brown, T.T.; Huggins, D.R. Lidar based biomass and crop nitrogen estimates for rapid, non-destructive assessment of wheat nitrogen status. Field Crop. Res. 2014, 159, 21–32. [Google Scholar] [CrossRef]
- Jin, X.; Kumar, L.; Li, Z.; Xu, X.; Yang, G.; Wang, J. Estimation of winter wheat biomass and yield by combining the AquaCrop model and field hyperspectral data. Remote Sens. 2016, 8, 972. [Google Scholar] [CrossRef]
- Wiseman, G.; McNairn, H.; Homayouni, S.; Shang, J.L. Radarsat-2 polarimetric SAR response to crop biomass for agricultural production monitoring. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4461–4471. [Google Scholar] [CrossRef]
- Li, F.; Etc, Y.M.; Li, F. Estimating winter wheat biomass and nitrogen status using an active crop sensor. Intell. Autom. Soft Comput. 2010, 16, 1221–1230. [Google Scholar]
- Wang, L.A.; Zhou, X.; Zhu, X.; Dong, Z.; Guo, W. Estimation of biomass in wheat using random forest regression algorithm and remote sensing data. Crop J. 2016, 4, 212–219. [Google Scholar] [CrossRef]
- Prabhakara, K.; Hively, W.D.; McCarty, G.W. Evaluating the relationship between biomass, percent groundcover and remote sensing indices across six winter cover crop fields in Maryland, United States. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 88–102. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with crop circle multispectral active canopy sensor. Field Crop. Res. 2014, 154, 133–144. [Google Scholar] [CrossRef]
- Kanke, Y.; Tubaña, B.; Dalen, M.; Harrell, D. Evaluation of red and red-edge reflectance-based vegetation indices for rice biomass and grain yield prediction models in paddy fields. Precis. Agric. 2016, 17, 507–530. [Google Scholar] [CrossRef]
- Kross, A.; Mcnairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of rapideye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Bareth, G.; Li, F.; Lenz-Wiedemann, V.I.S.; Koppe, W.; Miao, Y.; Hennig, S.D.; Jia, L.; Laudien, R.; Chen, X.; et al. Development and implementation of a multiscale biomass model using hyperspectral vegetation indices for winter wheat in the north China plain. Int. J. Appl. Earth Obs. Geoinf. 2014, 33, 232–242. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.X.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.K.; Huang, S.Y.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crop. Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Marshall, M.; Thenkabail, P. Advantage of hyperspectral EO-1 Hyperion over multispectral IKONOS, GeoEye-1, WorldView-2, Landsat ETM+, and MODIS vegetation indices in crop biomass estimation. ISPRS J. Photogramm. Remote Sens. 2015, 108, 205–218. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.P.; van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance to estimate genetic variation for in-season biomass, leaf chlorophyll, and canopy temperature in wheat. Crop Sci. 2006, 46, 1046. [Google Scholar] [CrossRef]
- Stroppiana, D.; Boschetti, M.; Brivio, P.A.; Bocchi, S. Plant nitrogen concentration in paddy rice from field canopy hyperspectral radiometry. Field Crop. Res. 2009, 111, 119–129. [Google Scholar] [CrossRef]
- Inoue, Y.; Guerif, M.; Baret, F.; Skidmore, A.; Gitelson, A.; Schlerf, M.; Darvishzadeh, R.; Olioso, A. Simple and robust methods for remote sensing of canopy chlorophyll content: A comparative analysis of hyperspectral data for different types of vegetation. Plant Cell Environ. 2016, 39, 2609–2623. [Google Scholar] [CrossRef] [PubMed]
- le Maire, G.; François, C.; Dufrêne, E. Towards universal broad leaf chlorophyll indices using prospect simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Féret, J.B.; François, C.; Gitelson, A.; Asner, G.P.; Barry, K.M.; Panigada, C.; Richardson, A.D.; Jacquemoud, S. Optimizing spectral indices and chemometric analysis of leaf chemical properties using radiative transfer modeling. Remote Sens. Environ. 2011, 115, 2742–2750. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J.; Hao, X.; Hunt, E.R., Jr. Estimating dry matter content from spectral reflectance for green leaves of different species. Int. J. Remote Sens. 2011, 32, 7097–7109. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. In Third Earth Resources Technology Satellite-1 Symposium-Volume I: Technical Presentations; NASA SP-351; NASA: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Huete, A.R.; Liu, H.Q.; Batchily, K.; Leeuwen, W.V. A comparison of vegetation indices over a global set of tm images for eos-modis. Remote Sens. Environ. 1997, 59, 440–451. [Google Scholar] [CrossRef]
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data : Decomposing biochemical from structural signals. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]
- le Maire, G.; Francois, C.; Soudani, K.; Berveiller, D.; Pontailler, J.; Breda, N.; Genet, H.; Davi, H.; Dufrene, E. Calibration and validation of hyperspectral indices for the estimation of broadleaved forest leaf chlorophyll content, leaf mass per area, leaf area index and leaf canopy biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Swain, K.C.; Thomson, S.J.; Jayasuriya, H.P.W. Adoption of an unmanned helicopter for low-altitude remote sensing to estimate yield and total biomass of a rice crop. Trans. ASAE 2010, 53, 21–27. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Ata-Ul-Karim, S.T.; Yao, X.; Liu, X.J.; Cao, W.X.; Zhu, Y. Development of critical nitrogen dilution curve of japonica rice in yangtze river reaches. Field Crop. Res. 2013, 149, 149–158. [Google Scholar] [CrossRef]
- Yao, X.; Ata-Ul-Karim, S.T.; Zhu, Y.; Tian, Y.C.; Liu, X.J.; Cao, W.X. Development of critical nitrogen dilution curve in rice based on leaf dry matter. Eur. J. Agron. 2014, 55, 20–28. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.-H.; Gastal, F. Diagnosis tool for plant and crop n status in vegetative stage theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Yang, J.C.; Peng, S.B.; Zhang, Z.J.; Wang, Z.Q.; Visperas, R.M.; Zhu, Q.S. Grain and dry matter yields and partitioning of assimilates in japonica/indica hybrid rice. Crop Sci. 2002, 42, 766–772. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P. Sentinel-2: ESA’s optical high-resolution mission for GMES operational services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. Evaluation of the MERIS terrestrial chlorophyll index (MTCI). Adv. Space Res. 2007, 39, 100–104. [Google Scholar] [CrossRef]
- Guanter, L.; Kaufmann, H.; Segl, K.; Foerster, S.; Rogass, C.; Chabrillat, S.; Kuester, T.; Hollstein, A.; Rossner, G.; Chlebek, C.; et al. The EnMAP spaceborne imaging spectroscopy mission for earth observation. Remote Sens. 2015, 7, 8830–8857. [Google Scholar] [CrossRef]
- Lee, C.M.; Cable, M.L.; Hook, S.J.; Green, R.O.; Ustin, S.L.; Mandl, D.J.; Middleton, E.M. An introduction to the NASA Hyperspectral InfraRed Imager (HyspIRI) mission and preparatory activities. Remote Sens. Environ. 2015, 167, 6–19. [Google Scholar] [CrossRef]





| Year | Early Tillering | Late Tillering | Jointing | Early Booting | Late Booting | Heading | Early Filling | Late Filling |
|---|---|---|---|---|---|---|---|---|
| 2014 | 10 July | 20 July | 30 July | / | 21 August | 2 September | / | 21 September |
| 2015 | 10 July | 22 July | 30 July | 14 August | 26 August | / | 9 September | 27 September |
| Canopy Component | No. of Samples | Mean ± SD | Minimum | Maximum | Growth Stage |
|---|---|---|---|---|---|
| Leaf | 359 | 1.66 ± 1.33 | 0.04 | 6.75 | All stages |
| Stem | 359 | 3.30 ± 2.93 | 0.07 | 12.54 | All stages |
| Panicle | 96 | 5.09 ± 3.11 | 0.83 | 12.80 | Post-heading |
| Leaf + stem | 359 | 4.95 ± 4.15 | 0.11 | 17.84 | All stages |
| Leaf + stem + panicle (Total) | 359 | 6.32 ± 5.96 | 0.11 | 25.94 | All stages |
| Index | Formulation | Reference |
|---|---|---|
| Red-edge Chlorophyll Index | [36] | |
| Ratio of Transformed Chlorophyll Absorption in Reflectance Index to Optimized Soil-Adjusted Vegetation Index | [37] | |
| Normalized Difference Vegetation Index | [38] | |
| Enhanced Vegetation Index | [39] | |
| Normalized Difference index for LMA * | [33] | |
| Normalized Dry Matter Index | [34] | |
| Normalized Difference Lignin Index | [40] | |
| Normalized Difference Index for leaf canopy biomass | [41] |
| Vegetation Index | Leaf Biomass (t/ha) | Stem Biomass (t/ha) | Leaf + Stem Biomass (t/ha) | Total Biomass (t/ha) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Linear | Nonlinear | Linear | Nonlinear | Linear | Nonlinear | Nonlinear | Linear (Before Heading) | Linear (After Heading) | |
| NDVI | 0.53 | 0.76 | 0.44 | 0.68 | 0.49 | 0.72 | 0.68 | 0.47 | 0.16 |
| EVI | 0.60 | 0.68 | 0.47 | 0.60 | 0.54 | 0.63 | 0.59 | 0.56 | 0.19 |
| CIRed-edge | 0.82 | 0.67 | 0.55 | 0.56 | 0.66 | 0.60 | 0.51 | 0.81 | 0.12 *** |
| TCARI/OSAVI | 0.58 | 0.76 | 0.38 | 0.61 | 0.46 | 0.66 | 0.56 | 0.55 | 0.06 * |
| NDLMA | 0.68 | 0.72 | 0.76 | 0.81 | 0.77 | 0.79 | 0.81 | 0.75 | 0.46 |
| NDMI | 0.68 | 0.70 | 0.63 | 0.71 | 0.68 | 0.72 | 0.69 | 0.68 | 0.25 |
| NDLI | 0.49 | 0.58 | 0.33 | 0.48 | 0.39 | 0.52 | 0.46 | 0.45 | 0.07 ** |
| NDBleaf | 0.44 | 0.46 | 0.41 | 0.47 | 0.44 | 0.48 | 0.49 | 0.49 | 0.09 ** |
| Vegetation Index | Leaf Biomass (t/ha) | Stem Biomass (t/ha) | Leaf + Stem Biomass (t/ha) | Total Biomass (t/ha) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Linear | Nonlinear | Linear | Nonlinear | Linear | Nonlinear | Nonlinear | Linear (Before Heading) | Linear (After Heading) | |
| NDVI | 0.92 | 0.77 | 2.20 | 2.13 | 2.96 | 2.71 | 4.79 | 2.55 | 3.95 |
| EVI | 0.84 | 0.95 | 2.13 | 2.60 | 2.82 | 3.45 | 5.65 | 2.31 | 3.92 |
| CIRed-edge | 0.56 | 1.68 | 1.98 | 4.25 | 2.42 | 5.89 | 8.29 | 1.51 | 4.04 |
| TCARI/OSAVI | 0.87 | 0.73 | 2.32 | 2.35 | 3.06 | 2.96 | 5.48 | 2.35 | 4.18 |
| NDLMA | 0.75 | 0.76 | 1.45 | 1.49 | 1.99 | 2.03 | 3.07 | 1.75 | 3.19 |
| NDMI | 0.75 | 0.75 | 1.78 | 2.13 | 2.34 | 2.71 | 4.89 | 1.96 | 3.72 |
| NDLI | 0.95 | 1.06 | 2.41 | 2.85 | 3.24 | 3.81 | 6.11 | 2.59 | 4.18 |
| NDBleaf | 1.00 | 2.88 | 2.27 | 9.78 | 3.12 | 12.28 | 19.98 | 2.49 | 4.57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.; Song, R.; Li, D.; Zhou, K.; Zheng, H.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Spectroscopic Estimation of Biomass in Canopy Components of Paddy Rice Using Dry Matter and Chlorophyll Indices. Remote Sens. 2017, 9, 319. https://doi.org/10.3390/rs9040319
Cheng T, Song R, Li D, Zhou K, Zheng H, Yao X, Tian Y, Cao W, Zhu Y. Spectroscopic Estimation of Biomass in Canopy Components of Paddy Rice Using Dry Matter and Chlorophyll Indices. Remote Sensing. 2017; 9(4):319. https://doi.org/10.3390/rs9040319
Chicago/Turabian StyleCheng, Tao, Renzhong Song, Dong Li, Kai Zhou, Hengbiao Zheng, Xia Yao, Yongchao Tian, Weixing Cao, and Yan Zhu. 2017. "Spectroscopic Estimation of Biomass in Canopy Components of Paddy Rice Using Dry Matter and Chlorophyll Indices" Remote Sensing 9, no. 4: 319. https://doi.org/10.3390/rs9040319
APA StyleCheng, T., Song, R., Li, D., Zhou, K., Zheng, H., Yao, X., Tian, Y., Cao, W., & Zhu, Y. (2017). Spectroscopic Estimation of Biomass in Canopy Components of Paddy Rice Using Dry Matter and Chlorophyll Indices. Remote Sensing, 9(4), 319. https://doi.org/10.3390/rs9040319