4.1. Characteristics and Dynamics of Vegetation Indices of Saltbush and Annual Pasture
Overall, saltbush showed higher canopy coverage and higher biomass than pasture in both seasons, and the spectral signatures and the derived vegetation indices allowed discrimination between the two vegetation types from the images. These differences were particularly marked in the red band, with differences of the red band between pasture and saltbush of 29.5% in the green season and 34.4% in the dry season. It is thus possible to successfully distinguish pasture and saltbush canopy with an object-based classification method. Due to the senescence of pasture in the dry season, the physical, visual, and spectral differences between saltbush and pasture were much more pronounced in the images than in the green season, thus the dry season is the best time in this Mediterranean climate for vegetation classification. This difference has implications for future design of vegetation monitoring using image data.
Although nearly 90% of the planted saltbush had survived during the study time frame, the values of fc were moderate in both the green season (0.60) and dry season (0.54), which indicates that a greater proportion of the plots were covered by pasture and bare soil than by saltbush. This was confirmed by visual examination of the images. The low vegetation coverage and high salinity (EM38 H ranging from 50 to 300 mS m
−1) [
7] at the field sites resulted in generally low values of the NIR-based vegetation indices in the study area [
52]. For example, the mean NDVI of saltbush was 0.28 in the dry season and 0.26 in the green season, while the mean RVI was 1.9 and 1.8 in the same period.
4.2. Indicators of Carbon Stocks (Ct)
It can be concluded from the Spearman’s rank correlation test of vegetation indices against sequestered carbon (C) that vegetation indices calculated from red and NIR bands can accurately reflect the carbon storage for saltbush both at the individual plant and plot scale (
Table 3 and
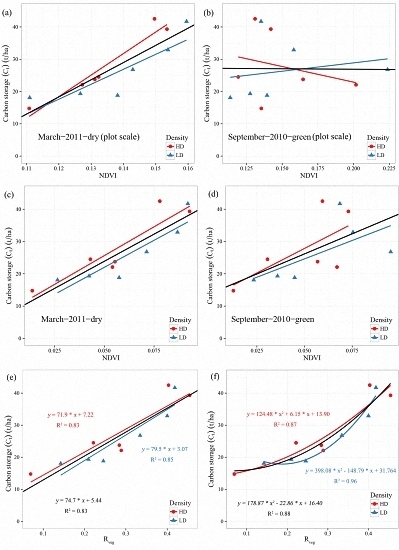
Table 4) in the dry season (March-2011-Dry image). However, in the green season (September-2010-Green image), carbon storage could only be suitably estimated at the individual plant scale, as the pasture in the green season was still alive (NDVI values of around 0.13), which dramatically changed the estimated total carbon storage for each plot. Meanwhile, only GCC and RVI showed reasonable results for estimating carbon of saltbush in the green season, again indicating that the best time for estimating saltbush biomass with remote sensing data is in the dry season.
GCC was significantly related to carbon storage of saltbush at the individual plant scale in both the dry and green seasons, which suggests that indices derived from bands only in the visible part of the electromagnetic spectrum, and without including a NIR band, can be a useful indicator of saltbush biomass. GCC has also been found to be a good indicator of vegetation health and phenology in other studies [
18,
40], as GCC best represented differences in healthy vigour and mortality of vegetation. Meanwhile, from the different relationship of GCC between plot and individual plot scales, it can be concluded that the best performance of GCC requires vegetation classification, suggesting that model precision is determined by the accuracy of vegetation classification. In addition, there was only a small difference between the Pasture and Saltbush values of GCC, with these showing a considerable contribution to model performance at plot scale.
Vegetation coverage indices (fc and Rveg) were strongly correlated to Ct at both the individual plant and plot scale, suggesting that canopy coverage of saltbush inherently reflects carbon storage. In addition, Rveg in this study area produced a better result than fc. The calculation of fc required the NDVI values of pure soil and vegetation, which is a source of additional uncertainty in the index. In this study, mean NDVI values from pure soil and vegetation samples within the image were used for calculating fc. However, there is still a high variation on both vegetation structure and soil properties. In order to increase the accuracy of fc, a spatial interpolation method could be used to predict variations in the spectral characteristics of bare soil and green vegetation across space.
The results for the R
veg are consistent with that of Suganuma et al. [
25] who used remote sensing derived canopy coverage to estimate stand biomass in forest species (
Acacia aneura and
Eucalyptus camaldulensis) in arid Western Australia. Similarly, Sousa et al. [
27], working on
Quercus rotundifolia in southern Portugal, found that AGB as a function of crown horizontal projection had the same trend for individual trees and plots, even though estimation for individual trees produced large individual errors. For our study, the strong relationship between vegetation coverage and C
t can possibly be explained by
A. nummularia having little variation in height due to the consistency in age and the strong relationship between diameter and biomass reported by Walden et al. [
11]. This is in contrast to many forest inventory studies where there is canopy closure and, thus, it is not possible to differentiate between individual trees and height has a large contribution to overall tree mass. For both this study and that of Suganuma et al. [
25], the canopies were separated, thus we can suggest that canopy coverage approaches may be applicable to carbon inventory in open woodlands as well as shrubby systems. Similar relationships between canopy coverage and biomass have also been reported in the semiarid savanna of Sudan [
53], and in semi-arid Senegal [
54]. However, different vegetation types showed significantly different estimation accuracy [
26].
For other vegetation indices in our study (i.e., NDVI, SAVI and RVI) that are derived from red and NIR bands, similar strong relationships with carbon storage of saltbush were observed at both individual plant and plot scales. This similarity may be because of their use of the same spectral bands (
Table 1). Both NDVI and RVI have been widely used for estimating AGB [
17,
23,
43,
55,
56].
Linear regression has been widely used to build the relationship between vegetation indices and carbon stocks, which demonstrates a satisfactory performance for carbon estimation. Overall, the linear regression models indicated strong relationships between the vegetation indices and carbon stocks, explaining around 80% of the variation, while the exponential function models explained around 85% of the variation (
Table A2). However, in this study, the exponential function and polynomial function models showed much better accuracy than the linear model in the comparison with different densities (
Figure 5). Similarly, other studies found close relationships between RVI and AGB with power and exponential functions [
57]. A power function model was also found for grassland [
21,
48]. Furthermore, Santin-Janin et al. [
58] developed a generalized non-linear model for the relationship between biomass and NDVI for
Acaena magellanica and
Taraxacum officinale. Meanwhile, as for the sensitivity of vegetation indices to planting density, a slightly stronger relationship was found in low density plots (R
2 = 0.96,
p ~ 0.01) than in high density plots (R
2 ~ 0.90,
p ~ 0.01) at the individual plant scale (
Figure 5 and
Figure A2), but at the plot scale, a much higher difference occurred (
Figure A1). This difference between the low and high density plots can be ascribed to the effects of a higher ratio of pasture in the low density plots than in high density plots. In addition to the effects of Pasture at plot scale, the accuracy of vegetation classification can be another factor inducing a different relationship between low and high density plots. The difference of the best-fit regression model between high and low density plots also resulted from the different canopy coverage of each plant. Generally, low density plots have a higher canopy coverage (an average of 2.66 × 2.80 m
2) than those in high density plots (an average of 1.51 × 1.56 m
2) [
7], which resulted in the different performance of each vegetation index on carbon estimation. Besides, carbon storage can be different even for the same canopy coverage because of the difference in height of plants between low (an average of 2.1 m) and high density (an average of 1.7 m) plots.
4.3. Limitations and Future Research
The object-based classification method was successfully used to distinguish pasture and saltbush from the high resolution image data. Although the efficacy of the technique was demonstrated here at a single location, the underlying allometric equation between saltbush carbon yield and stand parameters had been calibrated at six sites across southern Australia [
11], and this suggests that our results are broadly applicable across other regions.
The mean classification stability and best classification results were 0.7 and 0.85, respectively, but there is still uncertainty related to the identification of the boundary of each saltbush plant. Although the annual pastures had died/senesced by the time the dry season image was acquired, it was still difficult to distinguish the boundary of each saltbush due to its overall low coverage (approximate fc of 0.17 to 0.69). Moreover, compared to the size of the saltbush canopy (an average of 1.79 × 1.85 m2), the pixel size of our image (0.5 m) is still relatively coarse, which makes the pixels in the boundary area to be a mixture of both soil background and saltbush branches, especially in high density plots. Therefore, it is impossible to find a fixed threshold to distinguish saltbush and soil. The spectral response from saltbush in some pixels may be confounded by that from the soil background, and saltbush pixels could therefore be misclassified as pasture during the classification process. Therefore, the potential use of images with finer pixels should enhance the accuracy of remotely sensed data in the future.
The relationships between remote sensing indices and carbon storage will vary in relation to the site-specific properties of soil condition, shadow, different species, and canopy structure. Meanwhile, soil background also has high spatial and seasonal variation. Therefore, the regression quality reported by previous studies varies strongly, R2 with a range of 0.32 to 0.95 and our relationships may only be suitable for similar climatic and vegetation types as in the study, especially as the assumption of a set root mass to canopy relationship is inherent in our calibration data. However, our findings do demonstrate the capability of this approach to estimate carbon stocks using high-resolution remote sensing images in vegetation with non-overlapping canopies.
The applicability of our results could be further validated in other regions where abandoned farmland is being revegetated to ameliorate negative impacts of agricultural practices. With the advent of unmanned aerial vehicle (UAV) technology, there is the potential to gain significantly higher resolution imagery at a much lower cost (ca. USD
$4000 for a DJI Phantom 4 Pro and a NDVI supported camera [
59]) and with far greater flexibility of application and hence the rapid and cheap assessment of carbon. Recent examples of sensors mounted on UAV have included pixel resolutions as fine as 0.01 m, which provides sufficiently detailed information for estimating biomass of crops and monitoring forests [
60]. The technical specifications of sensors mounted on UAV clearly have the potential to be used for monitoring biomass of vegetation used for carbon stocks, but the design of such a monitoring system has additional requirements, such as determining the best seasonal timing of measurements and assessing the potential for monitoring at the tree- or stand-scale.
With the possibility of finer resolution images for monitoring vegetation, remote sensing methodologies could potentially deliver estimates of biomass with greater precision and accuracy, as more accurate classification results are likely to be achieved for canopy classification with the Objective-based classification method. Finally, as canopy coverage and vegetation indices show high accuracy for estimating carbon stocks at the plot scale, some frequently used sources of image data, for example, Landsat-TM and SPOT, which are of medium spatial resolution and can provide an estimation of canopy coverage, may also be useful for broad scale biomass estimation.