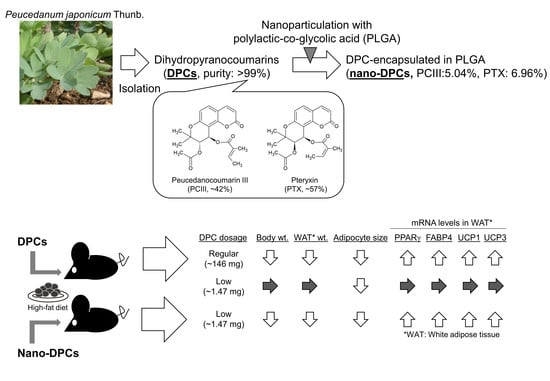
Dihydropyranocoumarins Exerted Anti-Obesity Activity In Vivo and its Activity Was Enhanced by Nanoparticulation with Polylactic-Co-Glycolic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purification of DPCs from PJT and Preparation of Nano-DPCs
2.2. Animals
2.3. Lipid Concentrations and Biochemical Parameters in the Serum, Liver, and Feces
2.4. Adipocyte Size
2.5. Quantitative Real-Time Polymerase Chain Reaction
2.6. Ultraperformance Liquid Chromatography Analysis of DPCs Concentration in Epididymal WAT
2.7. Statistical Analyses
3. Results
3.1. DPC Content in Purified DPCs and Nano-DPCs
3.2. Effect of Dietary DPCs and Nano-DPCs Administration on Growth Parameters
3.3. Effect of Dietary DPCs and Nano-DPCs Administration on Serum and Hepatic Parameters
3.4. Effects of DPCs and Nano-DPCs on Lipid Accumulation in Epididymal Adipose Tissues
3.5. Effect of Dietary DPCs and Nano-DPCs Administration on Lipid Metabolism–Related Gene Expressions in Epididymal WAT
3.6. Effect of PLGA Nanoparticulation on the Concentration of DPCs in WAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barja-Fernandez, S.; Leis, R.; Casanueva, F.F.; Seoane, L.M. Drug development strategies for the treatment of obesity: How to ensure efficacy, safety, and sustainable weight loss. Drug Des. Dev. 2014, 8, 2391–2400. [Google Scholar] [CrossRef] [Green Version]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.T.; Somoza, V. Extracellular Vesicles as Vehicles for the Delivery of Food Bioactives. J. Agric. Food Chem. 2019, 67, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Nanotechnology Applications in Functional Foods; Opportunities and Challenges. Prev. Nutr. Food Sci. 2016, 21, 1–8. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- Zakeri-Milani, P.; Loveymi, B.D.; Jelvehgari, M.; Valizadeh, H. The characteristics and improved intestinal permeability of vancomycin PLGA-nanoparticles as colloidal drug delivery system. Colloids Surf. B Biointerfaces 2013, 103, 174–181. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, P.-Y.; Wu, C.-C.; Chen, I.-S. Chemical constituents and anti-platelet aggregation activity from the root of Peucedanum formosanum. J. Food Drug Anal. 2008, 16, 15–25. [Google Scholar]
- Aida, Y.; Kasama, T.; Takeuchi, N.; Chiba, M.; Tobinaga, S. Pharmacological activities of khellactones, compounds isolated from Peucedanum japonicum THUNB. and Peucedanum praeruptorium DUNN. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 343–352. [Google Scholar] [CrossRef]
- Sarkhail, P.; Shafiee, A.; Sarkheil, P. Biological activities and pharmacokinetics of praeruptorins from Peucedanum species: A systematic review. Biomed. Res. Int. 2013, 2013, 343808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesla, L.; Skalicka-Wozniak, K.; Hajnos, M.; Hawryl, M.; Waksmundzka-Hajnos, M. Multidimensional TLC procedure for separation of complex natural mixtures spanning a wide polarity range; Application for fingerprint construction and for investigation of systematic relationships within the Peucedanum genus. Acta Chromatogr. 2009, 21, 641–657. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Los, R.; Glowniak, K.; Malm, A. Volatile compounds in fruits of Peucedanum cervaria (Lap.) L. Chem. Biodivers. 2009, 6, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Nugara, R.N.; Inafuku, M.; Takara, K.; Ogi, T.; Ichiba, T.; Iwasaki, H.; Okabe, T.; Oku, H. In vivo and in vitro anti-obesity activities of dihydropyranocoumarins derivatives from Peucedanum japonicum Thunb. J. Funct. Foods 2017, 29, 19–28. [Google Scholar] [CrossRef]
- Okabe, T.; Toda, T.; Nukitrangsan, N.; Inafuku, M.; Iwasaki, H.; Oku, H. Peucedanum japonicum Thunb inhibits high-fat diet induced obesity in mice. Phytother. Res. 2011, 25, 870–877. [Google Scholar] [CrossRef]
- Nukitrangsan, N.; Okabe, T.; Toda, T.; Inafuku, M.; Iwasaki, H.; Yanagita, T.; Oku, H. Effect of Peucedanum japonicum Thunb on the expression of obesity-related genes in mice on a high-fat diet. J. Oleo Sci. 2011, 60, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Chen, H.C.; Farese, R.V., Jr. Determination of adipocyte size by computer image analysis. J. Lipid Res. 2002, 43, 986–989. [Google Scholar]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, P.; Wafer, R.; Minchin, J.E.N. Adipose morphology and metabolic disease. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akazawa, S.; Sun, F.; Ito, M.; Kawasaki, E.; Eguchi, K. Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care 2000, 23, 1067–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [Green Version]
- Fajas, L.; Fruchart, J.C.; Auwerx, J. Transcriptional control of adipogenesis. Curr. Opin. Cell Biol. 1998, 10, 165–173. [Google Scholar] [CrossRef]
- Gustafson, B.; Gogg, S.; Hedjazifar, S.; Jenndahl, L.; Hammarstedt, A.; Smith, U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E999–E1003. [Google Scholar] [CrossRef] [Green Version]
- Iwase, M.; Yamamoto, T.; Nishimura, K.; Takahashi, H.; Mohri, S.; Li, Y.; Jheng, H.F.; Nomura, W.; Takahashi, N.; Kim, C.S.; et al. Suksdorfin Promotes Adipocyte Differentiation and Improves Abnormalities in Glucose Metabolism via PPARgamma Activation. Lipids 2017, 52, 657–664. [Google Scholar] [CrossRef]
- Palou, A.; Pico, C.; Bonet, M.L.; Oliver, P. The uncoupling protein, thermogenin. Int. J. Biochem. Cell Biol. 1998, 30, 7–11. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847. [Google Scholar] [CrossRef] [Green Version]
- Cinti, S. The adipose organ at a glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Almind, K.; Manieri, M.; Sivitz, W.I.; Cinti, S.; Kahn, C.R. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 2366–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Nair, H.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharm. 2010, 79, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Kozuka, C.; Shimizu-Okabe, C.; Takayama, C.; Nakano, K.; Morinaga, H.; Kinjo, A.; Fukuda, K.; Kamei, A.; Yasuoka, A.; Kondo, T.; et al. Marked augmentation of PLGA nanoparticle-induced metabolically beneficial impact of gamma-oryzanol on fuel dyshomeostasis in genetically obese-diabetic ob/ob mice. Drug Deliv. 2017, 24, 558–568. [Google Scholar] [CrossRef] [Green Version]







| Experimental Group | ||||
|---|---|---|---|---|
| Control | Regular Dose DPC | Low Dose DPC | Nano-DPC | |
| Total DPC dosage (mg) § | ||||
| by diet | 0 | 146 ± 1 | 147 ± 0 | 0 |
| by gavage | 0 | 0 | 0 | 1.48 ± 0 |
| Oral administration by gavage (µg/injection) † | ||||
| Nano-DPC | 0 | 0 | 0 | 615 ‡ |
| Vehicle PLGA nanoparticles | 541 | 541 | 541 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossin, A.Y.; Inafuku, M.; Oku, H. Dihydropyranocoumarins Exerted Anti-Obesity Activity In Vivo and its Activity Was Enhanced by Nanoparticulation with Polylactic-Co-Glycolic Acid. Nutrients 2019, 11, 3053. https://doi.org/10.3390/nu11123053
Hossin AY, Inafuku M, Oku H. Dihydropyranocoumarins Exerted Anti-Obesity Activity In Vivo and its Activity Was Enhanced by Nanoparticulation with Polylactic-Co-Glycolic Acid. Nutrients. 2019; 11(12):3053. https://doi.org/10.3390/nu11123053
Chicago/Turabian StyleHossin, Abu Yousuf, Masashi Inafuku, and Hirosuke Oku. 2019. "Dihydropyranocoumarins Exerted Anti-Obesity Activity In Vivo and its Activity Was Enhanced by Nanoparticulation with Polylactic-Co-Glycolic Acid" Nutrients 11, no. 12: 3053. https://doi.org/10.3390/nu11123053
APA StyleHossin, A. Y., Inafuku, M., & Oku, H. (2019). Dihydropyranocoumarins Exerted Anti-Obesity Activity In Vivo and its Activity Was Enhanced by Nanoparticulation with Polylactic-Co-Glycolic Acid. Nutrients, 11(12), 3053. https://doi.org/10.3390/nu11123053