Probiotic Supplementation is Associated with Increased Antioxidant Capacity and Copper Chelation in C. difficile-Infected Fecal Water
Abstract
:1. Introduction
2. Materials and Methods
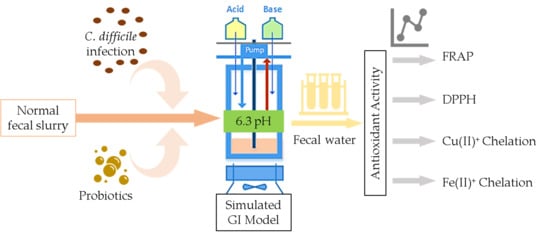
2.1. Batch Culture Fermentation
2.1.1. Fecal Slurry Preparation
2.1.2. Probiotic Treatment Preparation
2.1.3. Experimental Repeats
2.2. Antioxidant Assays
2.2.1. Chemical Reagents
2.2.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.2.3. 2’,2’-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.2.4. Cu (II)+ Chelation Assay
2.2.5. Fe (II)+ Chelation Assay
2.3. Statistical Analysis
3. Results and Discussion
3.1. FRAP and DPPH Antioxidant Capacity of Fecal Water
3.2. Metal Chelation Capability of Fecal Water
Determination of Copper Chelation
3.3. Determination of Nitrite, Nitrate, and Protein Carbonyls
3.4. Correlation of Antioxidant Capacity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, R.D. The Role of Probiotic Cultures in the Control of Gastrointestinal Health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Boirivant, M.; Strober, W. The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 2007, 23, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Probiotic therapy of intestinal inflammation and infections. Curr. Opin. Gastroenterol. 2005, 21, 44–50. [Google Scholar] [PubMed]
- Shah, C.; Mokashe, N.; Chavan, R.; Prajapati, J.; Mishra, V.; Yadav, H. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Mercenier, A.; Pavan, S.; Pot, B. Probiotics as biotherapeutic agents: Present knowledge and future prospects. Curr. Pharm. Des. 2002, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, H.; Koning, C.; Mulder, L.; Rombouts, F.; Beynen, A. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Yen, C.-L. Antioxidative Ability of Lactic Acid Bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Sikes, M.; Bruno-Bárcena, J.M. The intestinal microbiota, gastrointestinal environment and colorectal cancer: A putative role for probiotics in prevention of colorectal cancer? Am. J. Physiol. Liver Physiol. 2011, 301, G401–G424. [Google Scholar] [CrossRef]
- Garsetti, M.; Pellegrini, N.; Baggio, C.; Brighenti, F. Antioxidant activity in human faeces. Br. J. Nutr. 2007, 84, 705–710. [Google Scholar] [CrossRef]
- Thomson, A.; Hemphill, D.; Jeejeebhoy, K.N. Oxidative Stress and Antioxidants in Intestinal Disease. Dig. Dis. 1998, 16, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Reimund, J.M.; Allison, A.C.; Muller, C.D.; Dumont, S.; Kenney, J.S.; Baumann, R.; Duclos, B.; Poindron, P. Antioxidants inhibit the in vitro production of inflammatory cytokines in Crohn’s disease and ulcerative colitis. Eur. J. Clin. Investig. 1998, 28, 145–150. [Google Scholar] [CrossRef]
- Stone, W.L.; Papas, A.M. Tocopherols and the Etiology of Colon Cancer. J. Natl. Cancer Inst. 1997, 89, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.A.; Scazzina, F.; Del Rio, D.; Valtueña, S.; Pellegrini, N.; Franzini, L.; Callegari, M.L.; Pellacani, C.; Buschini, A.; Zavaroni, I.; et al. Ability of a high-total antioxidant capacity diet to increase stool weight and bowel antioxidant status in human subjects. Br. J. Nutr. 2010, 104, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Frädrich, C.; Beer, L.-A.; Gerhard, R. Reactive Oxygen Species as Additional Determinants for Cytotoxicity of Clostridium difficile Toxins A and B. Toxins 2016, 8, 25. [Google Scholar] [CrossRef]
- Farrow, M.A.; Chumbler, N.M.; Lapierre, L.A.; Franklin, J.L.; Rutherford, S.A.; Goldenring, J.R.; Lacy, D.B. Clostridium difficile toxin B-induced necrosis is mediated by the host epithelial cell NADPH oxidase complex. Proc. Natl. Acad. Sci. USA 2013, 110, 18674–18679. [Google Scholar] [CrossRef]
- He, D.; Hagen, S.; Pothoulakis, C.; Chen, M.; Medina, N.; Warny, M.; LaMont, J. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 2000, 119, 139–150. [Google Scholar] [CrossRef]
- Surawicz, C.M. Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans. Best Pr. Res. Clin. Gastroenterol. 2003, 17, 775–783. [Google Scholar] [CrossRef]
- Suryavanshi, A.; Kaler, A.; Bihade, U.; Kaur, J.; Tikoo, K.B.; Agarwal, A.; Banerjee, U.C. Comparative studies on the antioxidant potential of vanillin-producing Saccharomyces boulardii extracts. Oxid. Antioxidants Med. Sci. 2013, 2, 1. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.I.R.; McDougall, G.J.; Glidewell, S.; Stewart, D.; Shen, Q.; Tuohy, K.; Dobbin, A.; Boyd, A.; Brown, E.; Haldar, S.; et al. Profiling of Phenols in Human Fecal Water after Raspberry Supplementation. J. Agric. Food Chem. 2010, 58, 10389–10395. [Google Scholar] [CrossRef] [PubMed]
- Record, I.R.; McInerney, J.K.; Noakes, M.; Bird, A.R. Chocolate Consumption, Fecal Water Antioxidant Activity, and Hydroxyl Radical Production. Nutr. Cancer 2003, 47, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.R.; Gill, C.I.; Rowland, I.R. Diet, fecal water, and colon cancer—Development of a biomarker. Nutr. Rev. 2009, 67, 509–526. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, S.I.; Heo, T.R. Screening of antioxidative activity of Bifidobacterium species isolated from Korean infant feces and their identification. Biotechnol. Bioprocess Eng. 2003, 8, 199–204. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, K.-T.; Chung, M.-Y.; Cho, D.-H.; Park, C.-S. Resistance of Lactobacillus casei KCTC 3260 to Reactive Oxygen Species (ROS): Role for a Metal Ion Chelating Effect. J. Food Sci. 2005, 70, m388–m391. [Google Scholar] [CrossRef]
- Molly, K.; Woestyne, M.V.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of Surface Exopolysaccharides from Bifidobacterium and Lactobacillus Within the Intestinal Environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Effect of dairy probiotic combinations on in vitro gastrointestinal tolerance, intestinal epithelial cell adhesion and cytokine secretion. J. Funct. Foods 2014, 8, 18–25. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Datta, S.; Timson, D.J.; Annapure, U.S. Antioxidant properties and global metabolite screening of the probiotic yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 2017, 97, 3039–3049. [Google Scholar] [CrossRef]
- Ahire, J.J.; Mokashe, N.U.; Patil, H.J.; Chaudhari, B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2013, 50, 26–34. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S. Fenton chemistry. Amino acid oxidation. J. Biol. Chem. 1991, 266, 17201–17211. [Google Scholar]
- Kim, H.; Rhee, S.H.; Kokkotou, E.; Na, X.; Savidge, T.; Moyer, M.P.; Pothoulakis, C.; Lamont, J.T. Clostridium difficile Toxin A Regulates Inducible Cyclooxygenase-2 and Prostaglandin E2Synthesis in Colonocytes via Reactive Oxygen Species and Activation of p38 MAPK. J. Biol. Chem. 2005, 280, 21237–21245. [Google Scholar] [CrossRef]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Neumann, W.; Gulati, A.; Nolan, E.M. Metal homeostasis in infectious disease: Recent advances in bacterial metallophores and the human metal-withholding response. Curr. Opin. Chem. Biol. 2017, 37, 10–18. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
 Normal FW
Normal FW  C. difficile-infected FW. Values are shown as mean ± SEM. The symbol ∆ represents significant differences (p < 0.05) between treatment at a particular time point and blank at the corresponding time point. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
C. difficile-infected FW. Values are shown as mean ± SEM. The symbol ∆ represents significant differences (p < 0.05) between treatment at a particular time point and blank at the corresponding time point. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
 Normal FW
Normal FW  C. difficile-infected FW. Values are shown as mean ± SEM. The symbol ∆ represents significant differences (p < 0.05) between treatment at a particular time point and blank at the corresponding time point. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
C. difficile-infected FW. Values are shown as mean ± SEM. The symbol ∆ represents significant differences (p < 0.05) between treatment at a particular time point and blank at the corresponding time point. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
 Normal FW
Normal FW  C. difficile-infected FW. Values are shown as mean ± SEM. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11= L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
C. difficile-infected FW. Values are shown as mean ± SEM. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11= L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
 Normal FW
Normal FW  C. difficile-infected FW. Values are shown as mean ± SEM. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11= L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
C. difficile-infected FW. Values are shown as mean ± SEM. Means at time points within treatments without a common letter are significantly different (p < 0.05). LR11= L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii.
 Normal fecal sample;
Normal fecal sample;  C. difficile-infected fecal sample. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. (LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii).
C. difficile-infected fecal sample. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. (LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii).
 Normal fecal sample;
Normal fecal sample;  C. difficile-infected fecal sample. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. (LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii).
C. difficile-infected fecal sample. The symbol * represents significant differences between treatment and blank (p < 0.05) when the means of all time points are jointly considered. (LR11 = L. rhamnosus R0011; LH52 = L. helveticus R0052; LGG = L. rhamnosus GG; SB = S. boulardii; BL175 = B. longum R0175; PROTO = ProtecFlorTM; LR+LH+BL = combination of L. rhamnosus R0011, L. helveticus R0052 and B. longum R0175; LGG+SB = combination of L. rhamnosus GG and S. boulardii).




| Variables | FRAP | DPPH | Cu (II)+ Chelation | Fe (II)+ Chelation |
|---|---|---|---|---|
| FRAP | 1 p = n/a | |||
| DPPH | −0.0645 p = 0.4572 | 1 p = n/a | ||
| Cu (II)+ chelation | 0.3648 p = 0.0001 * | −0.1497 p = 0.0831 | 1 p = n/a | |
| Fe (II)+ chelation | −0.2289 p = 0.0076 * | 0.4149 p < 0.0001 * | −0.3044 p = 0.0116 (*) | 1 p = n/a |
| Variables | FRAP | DPPH | Cu (II)+ chelation | Fe (II)+ chelation |
|---|---|---|---|---|
| FRAP | 1 p = n/a | |||
| DPPH | 0.1201 p = 0.1653 | 1 p = n/a | ||
| Cu (II)+ chelation | 0.3069 p = 0.0003 * | 0.1187 p = 0.1704 | 1 p = n/a | |
| Fe (II)+ chelation | −0.0162 p = 0.8516 | 0.0822 p = 0.3429 | −0.2573 p = 0.0026 (*) | 1 p = n/a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaisawat, M.B.; Iskandar, M.M.; MacPherson, C.W.; Tompkins, T.A.; Kubow, S. Probiotic Supplementation is Associated with Increased Antioxidant Capacity and Copper Chelation in C. difficile-Infected Fecal Water. Nutrients 2019, 11, 2007. https://doi.org/10.3390/nu11092007
Gaisawat MB, Iskandar MM, MacPherson CW, Tompkins TA, Kubow S. Probiotic Supplementation is Associated with Increased Antioxidant Capacity and Copper Chelation in C. difficile-Infected Fecal Water. Nutrients. 2019; 11(9):2007. https://doi.org/10.3390/nu11092007
Chicago/Turabian StyleGaisawat, Mohd Baasir, Michèle M. Iskandar, Chad W. MacPherson, Thomas A. Tompkins, and Stan Kubow. 2019. "Probiotic Supplementation is Associated with Increased Antioxidant Capacity and Copper Chelation in C. difficile-Infected Fecal Water" Nutrients 11, no. 9: 2007. https://doi.org/10.3390/nu11092007
APA StyleGaisawat, M. B., Iskandar, M. M., MacPherson, C. W., Tompkins, T. A., & Kubow, S. (2019). Probiotic Supplementation is Associated with Increased Antioxidant Capacity and Copper Chelation in C. difficile-Infected Fecal Water. Nutrients, 11(9), 2007. https://doi.org/10.3390/nu11092007