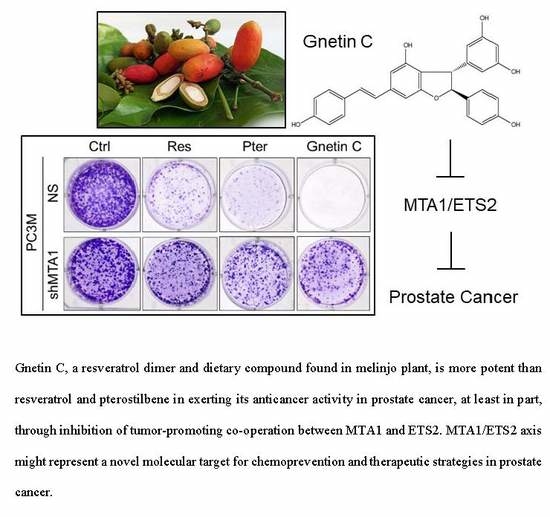
MTA1-Dependent Anticancer Activity of Gnetin C in Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Plasmids and Transfections
2.4. Western Blotting
2.5. qRT-PCR
2.6. MTT Assay
2.7. Flow Cytometry
2.8. Colony Formation Assay
2.9. Wound Healing Assay
2.10. Immunoprecipitation
2.11. Immunofluorescence
2.12. Statistical Analysis
3. Results
3.1. Gnetin C Inhibits MTA1 in PCa Cells More Potently than Resveratrol and Pterostilbene
3.2. Gnetin C Induces MTA1-Dependent Cytotoxicity in PCa Cells more Potently than Resveratrol and Pterostilbene
3.3. Gnetin C Inhibits MTA1-Dependent Metastatic Potential of PCa Cells More Potently than Resveratrol and Pterostilbene
3.4. MTA1 and ETS2 Exhibit Direct Positive Correlation in PCa
3.5. ETS2 Participates in MTA1-Mediated Functions in PCa
3.6. ETS2 Inhibition by Gnetin C in PCa Cells is More Potent than Resveratrol and Pterostilbene and is MTA1 Dependent
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Patterson, W., 3rd; Georgel, P.T. The epigenetic potentials of dietary polyphenols in prostate cancer management. Biochem. Cell Biol. 2013, 91, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P. Dietary botanicals for chemoprevention of prostate cancer. J. Tradit. Complement. Med. 2014, 4, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Crowe, K.M.; Allison, D.; Bioactive Food Components Conference Speakers. Evaluating bioactive food components in obesity and cancer prevention. Crit. Rev. Food Sci. Nutr. 2015, 55, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Aghajanpour, M.; Nazer, M.R.; Obeidavi, Z.; Akbari, M.; Ezati, P.; Kor, N.M. Functional foods and their role in cancer prevention and health promotion: A comprehensive review. Am. J. Cancer Res. 2017, 7, 740–769. [Google Scholar] [PubMed]
- Lopez-Guarnido, O.; Urquiza-Salvat, N.; Saiz, M.; Lozano-Paniagua, D.; Rodrigo, L.; Pascual-Geler, M.; Lorente, J.A.; Alvarez-Cubero, M.J.; Rivas, A. Bioactive compounds of the Mediterranean diet and prostate cancer. Aging Male 2018, 21, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.D.; Tapia, C.; Browne, T.J.; Mirlacher, M.; Sauter, G.; Rubin, M.A. Comprehensive analysis of the expression of the metastasis-associated gene 1 in human neoplastic tissue. Arch. Pathol. Lab. Med. 2006, 130, 989–996. [Google Scholar]
- Dias, S.J.; Zhou, X.; Ivanovic, M.; Gailey, M.P.; Dhar, S.; Zhang, L.; He, Z.; Penman, A.D.; Vijayakumar, S.; Levenson, A.S. Nuclear MTA1 overexpression is associated with aggressive prostate cancer, recurrence and metastasis in African Americans. Sci. Rep. 2013, 3, 2331. [Google Scholar] [CrossRef]
- Kai, L.; Wang, J.; Ivanovic, M.; Chung, Y.T.; Laskin, W.B.; Schulze-Hoepfner, F.; Mirochnik, Y.; Satcher, R.L., Jr.; Levenson, A.S. Targeting prostate cancer angiogenesis through metastasis-associated protein 1 (MTA1). Prostate 2011, 71, 268–280. [Google Scholar] [CrossRef]
- Levenson, A.S.; Kumar, A.; Zhang, X. MTA family of proteins in prostate cancer: Biology, significance, and therapeutic opportunities. Cancer Metastasis Rev. 2014, 33, 929–942. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, S.; Campanelli, G.; Butt, N.A.; Schallheim, J.M.; Gomez, C.R.; Levenson, A.S. MTA1 drives malignant progression and bone metastasis in prostate cancer. Mol. Oncol. 2018, 12, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Zhang, L.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Atfi, A.; Zhang, X.; Levenson, A.S. Dietary pterostilbene is a novel MTA1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget 2016, 7, 18469–18484. [Google Scholar] [CrossRef] [PubMed]
- Sementchenko, V.I.; Schweinfest, C.W.; Papas, T.S.; Watson, D.K. ETS2 function is required to maintain the transformed state of human prostate cancer cells. Oncogene 1998, 17, 2883–2888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, G.M.; Napoli, S.; Valentini, A.; Cavalli, F.; Watson, D.K.; Catapano, C.V. Triplex DNA-mediated downregulation of Ets2 expression results in growth inhibition and apoptosis in human prostate cancer cells. Nucleic Acids Res. 2004, 32, 4358–4367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foos, G.; Hauser, C.A. Altered Ets transcription factor activity in prostate tumor cells inhibits anchorage-independent growth, survival, and invasiveness. Oncogene 2000, 19, 5507–5516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Linn, D.E.; Penney, K.L.; Bronson, R.T.; Mucci, L.A.; Li, Z. Deletion of Interstitial Genes between TMPRSS2 and ERG Promotes Prostate Cancer Progression. Cancer Res. 2016, 76, 1869–1881. [Google Scholar] [CrossRef] [Green Version]
- Perner, S.; Demichelis, F.; Beroukhim, R.; Schmidt, F.H.; Mosquera, J.M.; Setlur, S.; Tchinda, J.; Tomlins, S.A.; Hofer, M.D.; Pienta, K.G.; et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006, 66, 8337–8341. [Google Scholar] [CrossRef]
- Shaikhibrahim, Z.; Ochsenfahrt, J.; Fuchs, K.; Kristiansen, G.; Perner, S.; Wernert, N. ERG is specifically associated with ETS-2 and ETV-4, but not with ETS-1, in prostate cancer. Int. J. Mol. Med. 2012, 30, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Mehra, R.; Tomlins, S.A.; Yu, J.; Cao, X.; Wang, L.; Menon, A.; Rubin, M.A.; Pienta, K.J.; Shah, R.B.; Chinnaiyan, A.M. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008, 68, 3584–3590. [Google Scholar] [CrossRef]
- Rostad, K.; Mannelqvist, M.; Halvorsen, O.J.; Oyan, A.M.; Bo, T.H.; Stordrange, L.; Olsen, S.; Haukaas, S.A.; Lin, B.; Hood, L.; et al. ERG upregulation and related ETS transcription factors in prostate cancer. Int. J. Oncol. 2007, 30, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Corey, E.; Vessella, R.L.; Lange, P.H.; True, L.D.; Huang, G.M.; Nelson, P.S.; Hood, L. Identification of differentially expressed prostate genes: Increased expression of transcription factor ETS-2 in prostate cancer. Prostate 1997, 30, 145–153. [Google Scholar] [CrossRef]
- Plotnik, J.P.; Hollenhorst, P.C. Interaction with ZMYND11 mediates opposing roles of Ras-responsive transcription factors ETS1 and ETS2. Nucleic Acids Res. 2017, 45, 4452–4462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, A.; Alshalalfa, M.; Davicioni, E.; Gupta, A.; Yegnasubramanian, S.; Wheelan, S.J.; Epstein, J.I.; De Marzo, A.M.; Lotan, T.L. ETS2 is a prostate basal cell marker and is highly expressed in prostate cancers aberrantly expressing p63. Prostate 2018, 78, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Do, P.M.; Varanasi, L.; Fan, S.; Li, C.; Kubacka, I.; Newman, V.; Chauhan, K.; Daniels, S.R.; Boccetta, M.; Garrett, M.R.; et al. Mutant p53 cooperates with ETS2 to promote etoposide resistance. Genes Dev. 2012, 26, 830–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollareddy, M.; Dimitrova, E.; Vallabhaneni, K.C.; Chan, A.; Le, T.; Chauhan, K.M.; Carrero, Z.I.; Ramakrishnan, G.; Watabe, K.; Haupt, Y.; et al. Regulation of nucleotide metabolism by mutant p53 contributes to its gain-of-function activities. Nat. Commun. 2015, 6, 7389. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Tu, H.; Kollareddy, M.; Pant, V.; Li, Q.; Zhang, Y.; Jackson, J.G.; Suh, Y.A.; Elizondo-Fraire, A.C.; Yang, P.; et al. Pla2g16 phospholipase mediates gain-of-function activities of mutant p53. Proc. Natl. Acad. Sci. USA 2014, 111, 11145–11150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, S.; Kumar, A.; Li, K.; Tzivion, G.; Levenson, A.S. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochim. Biophys. Acta 2015, 1853, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Kai, L.; Samuel, S.K.; Levenson, A.S. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int. J. Cancer 2010, 126, 1538–1548. [Google Scholar] [CrossRef]
- Li, K.; Dias, S.J.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Lewin, J.R.; Levenson, A.S. Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS ONE 2013, 8, e57542. [Google Scholar] [CrossRef]
- Dias, S.J.; Li, K.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Levenson, A.S. Trimethoxy-resveratrol and piceatannol administered orally suppress and inhibit tumor formation and growth in prostate cancer xenografts. Prostate 2013, 73, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Butt, N.A.; Kumar, A.; Dhar, S.; Rimando, A.M.; Akhtar, I.; Hancock, J.C.; Lage, J.M.; Pound, C.R.; Lewin, J.R.; Gomez, C.R.; et al. Targeting MTA1/HIF-1alpha signaling by pterostilbene in combination with histone deacetylase inhibitor attenuates prostate cancer progression. Cancer Med. 2017, 6, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; D’Silva, M.; Dholakia, K.; Levenson, A.S. In Vitro Anticancer Properties of Table Grape Powder Extract (GPE) in Prostate Cancer. Nutrients 2018, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Carrero, Z.I.; Kollareddy, M.; Chauhan, K.M.; Ramakrishnan, G.; Martinez, L.A. Mutant p53 protects ETS2 from non-canonical COP1/DET1 dependent degradation. Oncotarget 2016, 7, 12554–12567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Lin, S.Y.; Dhar, S.; Rimando, A.M.; Levenson, A.S. Stilbenes Inhibit Androgen Receptor Expression in 22Rv1 Castrate-resistant Prostate Cancer Cells. J. Med. Active Plants 2014, 3, 1–8. [Google Scholar]
- Dhar, S.; Kumar, A.; Gomez, C.R.; Akhtar, I.; Hancock, J.C.; Lage, J.M.; Pound, C.R.; Levenson, A.S. MTA1-activated Epi-microRNA-22 regulates E-cadherin and prostate cancer invasiveness. FEBS Lett. 2017, 591, 924–933. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, S.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Zhang, X.; Levenson, A.S. Epigenetic potential of resveratrol and analogs in preclinical models of prostate cancer. Ann. N. Y. Acad. Sci. 2015, 1348, 1–9. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Mehra, R.; Rhodes, D.R.; Cao, X.; Wang, L.; Dhanasekaran, S.M.; Kalyana-Sundaram, S.; Wei, J.T.; Rubin, M.A.; Pienta, K.J.; et al. Integrative molecular concept modeling of prostate cancer progression. Nat. Genet. 2007, 39, 41–51. [Google Scholar] [CrossRef]
- Kumar, R.; Wang, R.A.; Bagheri-Yarmand, R. Emerging roles of MTA family members in human cancers. Semin. Oncol. 2003, 30, 30–37. [Google Scholar] [CrossRef]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of Melinjo (Gnetum gnemon L.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef]
- Kunimasa, K.; Ohta, T.; Tani, H.; Kato, E.; Eguchi, R.; Kaji, K.; Ikeda, K.; Mori, H.; Mori, M.; Tatefuji, T.; et al. Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract suppresses multiple angiogenesis-related endothelial cell functions and tumor angiogenesis. Mol. Nutr. Food Res. 2011, 55, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.K.; Kunimasa, K.; Yamori, Y.; Mori, M.; Mori, H.; Nakamura, K.; Miller, G.; Manne, U.; Tiwari, A.K.; Narayanan, B. Antitumor activity of melinjo (Gnetum gnemon L.) seed extract in human and murine tumor models in vitro and in a colon-26 tumor-bearing mouse model in vivo. Cancer Med. 2015, 4, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Elbadry, M.I.; Taniwaki, M.; Harada, K.; Trung, L.Q.; Nakagawa, N.; Takami, A.; Ishiyama, K.; Yamauchi, T.; Takenaka, K.; et al. The simultaneous inhibition of the mTOR and MAPK pathways with Gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett. 2017, 400, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Inaoka, P.T. Gnetin-C and other resveratrol oligomers with cancer chemopreventive potential. Ann. N. Y. Acad. Sci. 2017, 1403, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Tatefuji, T.; Yanagihara, M.; Fukushima, S.; Hashimoto, K. Safety assessment of melinjo (Gnetum gnemon L.) seed extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2014, 67, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Kanai, Y.; Katagiri, M.; Watanabe, T.; Mori, A.; Ikuta, T.; Tani, H.; Fukushima, S.; Tatefuji, T.; Shirasawa, T. Melinjo (Gnetum gnemon L.) Seed Extract Decreases Serum Uric Acid Levels in Nonobese Japanese Males: A Randomized Controlled Study. Evid. Based Complement. Alternat Med. 2013, 2013, 589169. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Hikami, S.; Iizuna, S.; Yoshimatsu, M.; Asama, T.; Ota, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K.; Higaki, K. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (Gnetum gnemon L.) seed extract powder. J. Agric. Food Chem. 2014, 62, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, Y.; Suzuki, S.; Espinoza, J.L.; Vu Quang, L.; Enomoto, M.; Takasugi, S.; Nakamura, A.; Nakayama, T.; Tani, H.; Hanamura, I.; et al. Immunomodulatory and Metabolic Changes after Gnetin-C Supplementation in Humans. Nutrients 2019, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Akishita, M.; Tani, H.; Tatefuji, T.; Ogawa, S.; Iijima, K.; Eto, M.; Shirasawa, T.; Ouchi, Y. trans-Resveratrol in Gnetum gnemon protects against oxidative-stress-induced endothelial senescence. J. Nat. Prod. 2013, 76, 1242–1247. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Dholakia, K.; Sikorska, G.; Martinez, L.A.; Levenson, A.S. MTA1-Dependent Anticancer Activity of Gnetin C in Prostate Cancer. Nutrients 2019, 11, 2096. https://doi.org/10.3390/nu11092096
Kumar A, Dholakia K, Sikorska G, Martinez LA, Levenson AS. MTA1-Dependent Anticancer Activity of Gnetin C in Prostate Cancer. Nutrients. 2019; 11(9):2096. https://doi.org/10.3390/nu11092096
Chicago/Turabian StyleKumar, Avinash, Kshiti Dholakia, Gabriela Sikorska, Luis A. Martinez, and Anait S. Levenson. 2019. "MTA1-Dependent Anticancer Activity of Gnetin C in Prostate Cancer" Nutrients 11, no. 9: 2096. https://doi.org/10.3390/nu11092096
APA StyleKumar, A., Dholakia, K., Sikorska, G., Martinez, L. A., & Levenson, A. S. (2019). MTA1-Dependent Anticancer Activity of Gnetin C in Prostate Cancer. Nutrients, 11(9), 2096. https://doi.org/10.3390/nu11092096