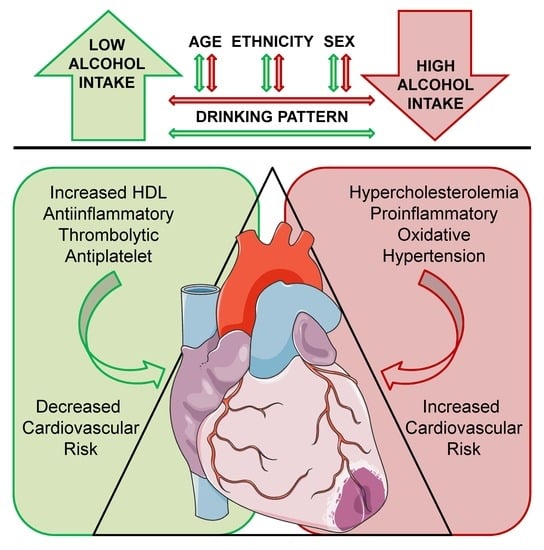
Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies
Abstract
:1. Introduction
2. Moderate versus Heavy Alcohol Intake
3. Acute versus Chronic Alcohol Consumption
4. Sex Differences
5. Ethnic Differences
6. Cardiovascular Disease
6.1. Intermediate Biomarkers of Cardiovascular Disease
6.2. Classical Cardiovascular Risk Factors
6.3. Major Adverse Cardiovascular Events
7. Cardiovascular and All-Cause Mortality
8. Type of Alcoholic Beverage Consumed
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ESC Cardiovascular Realities 2019 by-Flipsnack. Available online: https://www.flipsnack.com/Escardio/esc-cardiovascular-realities-2019/full-view.html (accessed on 21 October 2019).
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; Mcqueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ 2011, 342, d671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmot, M.; Brunner, E. Alcohol and cardiovascular disease. BMJ 1991, 303, 565. Available online: https://www.bmj.com/content/356/bmj.j1340.long (accessed on 6 November 2019). [CrossRef] [Green Version]
- Millwood, I.Y.; Walters, R.G.; Mei, X.W.; Guo, Y.; Yang, L.; Bian, Z.; Bennett, D.A.; Chen, Y.; Dong, C.; Hu, R.; et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: A prospective study of 500 000 men and women in China. Lancet 2019, 393, 1831–1842. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Status Report on Alcohol and Health 2018; WHO: Geneva, Italy, 2018. [Google Scholar]
- Cheng, Y.-C.; Huang, Y.-C.; Huang, W.-L. Heart rate variability as a potential biomarker for alcohol use disorders: A systematic review and meta-analysis. Drug Alcohol Depend. 2019, 204, 107502. [Google Scholar] [CrossRef]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.; Tymeson, H.D. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016-ClinicalKey. Lancet 2018, 392, 1015–1035. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S0140673618313102?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0140673618313102%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F (accessed on 22 October 2019). [CrossRef] [Green Version]
- Rehm, J.; Gmel, G.E.; Gmel, G.; Hasan, O.S.M.; Imtiaz, S.; Popova, S.; Probst, C.; Roerecke, M.; Room, R.; Samokhvalov, A.V.; et al. The relationship between different dimensions of alcohol use and the burden of disease—An update. Addiction 2017, 112, 968–1001. [Google Scholar] [CrossRef] [Green Version]
- 2015–2020 Dietary Guidelines for Americans; United States. Available online: https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf (accessed on 10 October 2019).
- Kalinowski, A.; Humphreys, K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction 2016, 111, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef]
- Mostofsky, E.; Chahal, H.S.; Mukamal, K.J.; Rimm, E.B.; Mittleman, M.A. Alcohol and immediate risk of cardiovascular events. Circulation 2016, 133, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guilleń, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D.; et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2012, 95, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martínez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef] [Green Version]
- Drinking Levels Defined; National Institute on Alcohol Abuse and Alcoholism (NIAAA): Bethesda, MD, USA, 2019. Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 6 November 2019).
- Jackson, R.; Broad, J.; Connor, J.; Wells, S. Alcohol and ischaemic heart disease: Probably no free lunch. Lancet 2005, 366, 1911–1912. [Google Scholar] [CrossRef]
- Tsubono, Y.; Yamada, S.; Nishino, Y.; Tsuji, I.; Hisamichi, S. Choice of comparison group in assessing the health effects of moderate alcohol consumption. Jama 2001, 286, 1177–1178. [Google Scholar] [CrossRef]
- Fillmore, K.M.; Stockwell, T.; Chikritzhs, T.; Bostrom, A.; Kerr, W. Moderate Alcohol Use and Reduced Mortality Risk: Systematic Error in Prospective Studies and New Hypotheses-ClinicalKey. Ann. Epidemiol. 2007, 17, S16–S23. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S1047279707000075?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1047279707000075%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F (accessed on 22 October 2019). [CrossRef]
- Britton, A.; Ben-Shlomo, Y.; Benzeval, M.; Kuh, D.; Bell, S. Life course trajectories of alcohol consumption in the United Kingdom using longitudinal data from nine cohort studies. BMC Med. 2015, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.-L.; Piano, M.R.; Thur, L.A.; Peters, T.A.; da Silva, A.L.G.; Phillips, S.A. The effects of repeated binge drinking on arterial stiffness and urinary norepinephrine levels in young adults. J. Hypertens. 2020, 38, 111–117. [Google Scholar] [CrossRef]
- Charakida, M.; Georgiopoulos, G.; Dangardt, F.; Chiesa, S.T.; Hughes, A.D.; Rapala, A.; Davey Smith, G.; Lawlor, D.; Finer, N.; Deanfield, J.E. Early vascular damage from smoking and alcohol in teenage years: The ALSPAC study. Eur. Heart J. 2019, 40, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Roerecke, M.; Rehm, J. Irregular heavy drinking occasions and risk of ischemic heart disease: A systematic review and meta-analysis. Am. J. Epidemiol. 2010, 171, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.; Fan, A.Z.; Freudenheim, J.L.; Dorn, J.; Trevisan, M. Lifetime drinking trajectories and nonfatal acute myocardial infarction. Alcohol. Clin. Exp. Res. 2019, 43, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldwater, D.; Karlamangla, A.; Merkin, S.S.; Seeman, T. Compared to non-drinkers, individuals who drink alcohol have a more favorable multisystem physiologic risk score as measured by allostatic load. PLoS ONE 2019, 14, e0223168. [Google Scholar] [CrossRef] [PubMed]
- Karlamangla, A.S.; Singer, B.H.; Seeman, T.E. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom. Med. 2006, 68, 500–507. [Google Scholar] [CrossRef]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; George, J.; Britton, A.; Bobak, M.; Casas, J.P.; Dale, C.E.; Denaxas, S.; Shah, A.D.; et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. BMJ 2017, 356, j909. [Google Scholar] [CrossRef] [Green Version]
- Puddey, I.B.; Mori, T.A.; Barden, A.E.; Beilin, L.J. Alcohol and hypertension—New insights and lingering controversies. Curr. Hypertens. Rep. 2019, 21, 79. [Google Scholar] [CrossRef]
- Rehm, J.; Roerecke, M. Cardiovascular effects of alcohol consumption. Trends Cardiovasc. Med. 2017, 27, 534–538. [Google Scholar] [CrossRef]
- Lévy, S.; Santini, L.; Capucci, A.; Oto, A.; Santomauro, M.; Riganti, C.; Raviele, A.; Cappato, R. European Cardiac Arrhythmia Society Statement on the cardiovascular events associated with the use or abuse of energy drinks. J. Interv. Card. Electrophysiol. 2019, 56, 99–115. [Google Scholar] [CrossRef]
- Matshipi, M.; Monyeki, K.D.; Mafumo, N.; Monyeki, S.M.; Siweya, H.J.; Kemper, H.C.G. The use of alcohol and knowledge of cardiovascular diseases among ellisras rural children aged 14–22 years: Ellisras longitudinal study. Int. J. Environ. Res. Public Health 2019, 16, 2650. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Solà, J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat. Rev. Cardiol. 2015, 12, 576–587. [Google Scholar] [CrossRef]
- Guzzo-Merello, G. Alcoholic cardiomyopathy. World J. Cardiol. 2014, 6, 771. [Google Scholar] [CrossRef]
- Russell, M.; Chu, B.C.; Banerjee, A.; Fan, A.Z.; Trevisan, M.; Dorn, J.M.; Gruenewald, P. Drinking patterns and myocardial infarction: A linear dose-response model. Alcohol. Clin. Exp. Res. 2009, 33, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Vatsalya, V.; Liaquat, H.B.; Ghosh, K.; Mokshagundam, S.P.; McClain, C.J. A review on the sex differences in organ and system pathology with alcohol drinking. Curr. Drug Abuse Rev. 2016, 9, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Ely, M. Gender differences in the relationship between alcohol consumption and drink problems are largely accounted for by body water. Alcohol Alcohol. 1999, 34, 894–902. [Google Scholar] [CrossRef]
- Thomasson, H.R. Gender differences in alcohol metabolism. In Recent Developments in Alcoholism; Springer: Boston, MA, USA, 2002; pp. 163–179. [Google Scholar]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.C.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Hear. J.-Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef] [Green Version]
- Urbano-Márquez, A. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA J. Am. Med. Assoc. 1995, 274, 149. [Google Scholar] [CrossRef]
- El-Mas, M.M.; Abdel-Rahman, A.A. Role of alcohol oxidative metabolism in its cardiovascular and autonomic effects. In Aldehyde Dehydrogenases; Springer: Singapore, 2019; pp. 1–33. [Google Scholar]
- Colditz, G.A.; Willett, W.C.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Menopause and the risk of coronary heart disease in women. N. Engl. J. Med. 1987, 316, 1105–1110. [Google Scholar] [CrossRef]
- Tolstrup, J.; Jensen, M.K.; Tjønneland, A.; Overvad, K.; Mukamal, K.J.; Grønbæk, M. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. Br. Med. J. 2006, 332, 1244–1247. [Google Scholar] [CrossRef] [Green Version]
- Schrieks, I.C.; Heil, A.L.J.; Hendriks, H.F.J.; Mukamal, K.J.; Beulens, J.W.J. The Effect of alcohol consumption on insulin sensitivity and glycemic status: A systematic review and meta-analysis of intervention studies. Diabetes Care 2015, 38, 723–732. [Google Scholar]
- Knott, C.; Bell, S.; Britton, A. Alcohol consumption and the risk of type 2 diabetes: A systematic review and Dose-Response Meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Mente, A.; Yusuf, S.; Gao, P.; Sleight, P.; Zhu, J.; Fagard, R.; Lonn, E.; Teo, K.K. Alcohol consumption and the risk of incident atrial fibrillation among people with cardiovascular disease. CMAJ 2012, 184, E857–E866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conen, D.; Tedrow, U.B.; Cook, N.R.; Moorthy, M.V.; Buring, J.E.; Albert, C.M. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA-J. Am. Med. Assoc. 2008, 300, 2489–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, L.; Vestergaard, P. Alcohol and risk of atrial fibrillation or flutter: a cohort study. Arch. Intern. Med. 2004, 164, 1993–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.C.; Drca, N.; Wolk, A. Alcohol consumption and risk of atrial fibrillation: A prospective study and dose-response meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 281–289. [Google Scholar] [CrossRef]
- Vallée, A.; Gabet, A.; Deschamps, V.; Blacher, J.; Olié, V. Relationship between nutrition and alcohol consumption with blood pressure: The ESTEBAN survey. Nutrients 2019, 11, 1433. [Google Scholar] [CrossRef] [Green Version]
- Di Castelnuovo, A.; Costanzo, S.; Bagnardi, V.; Donati, M.B.; Iacoviello, L.; De Gaetano, G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch. Intern. Med. 2006, 166, 2437–2445. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Lian, F.; Shi, Q.; Zhang, C.; Chen, Y.-W.; Zhou, Y.-H.; He, J. Alcohol intake and associated risk of major cardiovascular outcomes in women compared with men: A systematic review and meta-analysis of prospective observational studies. BMC Public Health 2015, 15, 773. [Google Scholar] [CrossRef] [Green Version]
- Cheung, J.W.; Cheng, E.P.; Wu, X.; Yeo, I.; Christos, P.J.; Kamel, H.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Ip, J.E.; et al. Sex-based differences in outcomes, 30-day readmissions, and costs following catheter ablation of atrial fibrillation: The United States Nationwide Readmissions Database 2010–14. Eur. Heart J. 2019, 40, 3035–3043. [Google Scholar]
- Suliga, E.; Kozieł, D.; Ciesla, E.; Rebak, D.; Głuszek-Osuch, M.; Naszydłowska, E.; Głuszek, S. The consumption of alcoholic beverages and the prevalence of cardiovascular diseases in men and women: A cross-sectional study. Nutrients 2019, 11, 1318. [Google Scholar] [CrossRef] [Green Version]
- Chaga, P.; Mazocco, L.; Piccoli, J.D.C.E.; Ardenghi, T.M.; Badimon, L.; Caramori, P.R.A.; Pellanda, L.; Gomes, I.; Schwanke, C.H.A. Association of alcohol consumption with coronary artery disease severity-ClinicalKey. Clin. Nutr. 2017, 36, 1036–1039. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S0261561416301510?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0261561416301510%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F (accessed on 29 October 2019). [CrossRef]
- Nansseu, J.R.; Kameni, B.S.; Assah, F.K.; Bigna, J.J.; Petnga, S.-J.; Tounouga, D.N.; Tchokfe Ndoula, S.; Noubiap, J.J.; Kamgno, J. Prevalence of major cardiovascular disease risk factors among a group of sub-Saharan African young adults: a population-based cross-sectional study in Yaoundé, Cameroon. BMJ Open 2019, 9, e029858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.P.; Xu, L.W.; Sun, T.; Wu, Y.Y.; Zhu, X.W.; Zhang, B.; Cheng, Z.; Cai, X.; Liu, Y.C.; Zhao, T.T.; et al. Relationship between alcohol use, blood pressure and hypertension: An association study and a Mendelian randomisation study. J. Epidemiol. Community Health 2019, 73, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Mulia, N.; Karriker-Jaffe, K.J.; Witbrodt, J.; Bond, J.; Williams, E.; Zemore, S.E. Racial/ethnic differences in 30-year trajectories of heavy drinking in a nationally representative U.S. sample. Drug Alcohol Depend. 2017, 170, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, P.; Nakayama, T.; Morita, A.; Sato, N.; Hishiki, M.; Saito, K.; Yoshikawa, Y.; Tamura, M.; Sato, I.; Takahashi, T.; et al. Common single nucleotide polymorphisms in Japanese patients with essential hypertension: aldehyde dehydrogenase 2 gene as a risk factor independent of alcohol consumption. Hypertens. Res. 2007, 30, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.C.; Greenfield, T.K.; Bond, J.; Ye, Y.; Rehm, J. Racial and ethnic differences in all-cause mortality risk according to alcohol consumption patterns in the national alcohol surveys. Am. J. Epidemiol. 2011, 174, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Sempos, C.T.; Rehm, J.; Wu, T.; Crespo, C.J.; Trevisan, M. Average volume of alcohol consumption and all-cause mortality in African Americans: The NHEFS cohort. Alcohol. Clin. Exp. Res. 2003, 27, 88–92. [Google Scholar] [CrossRef]
- O’Keefe, E.L.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Alcohol and cv health: Jekyll and Hyde J-Curves. Prog. Cardiovasc. Dis. 2018, 61, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, F.D.; Chambless, L.E.; Folsom, A.R.; Eigenbrodt, M.L.; Duncan, B.B.; Gilbert, A.; Szklo, M. Association between alcoholic beverage consumption and incidence of coronary heart disease in Whites and Blacks: The Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2004, 160, 466–474. [Google Scholar] [CrossRef]
- Taylor, B.; Irving, H.M.; Baliunas, D.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol and hypertension: Gender differences in dose-response relationships determined through systematic review and meta-analysis: REVIEW. Addiction 2009, 104, 1981–1990. [Google Scholar] [CrossRef]
- Holmes, M.V.; Dale, C.E.; Zuccolo, L.; Silverwood, R.J.; Guo, Y.; Ye, Z.; Prieto-Merino, D.; Dehghan, A.; Trompet, S.; Wong, A.; et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014, 349, g4164. [Google Scholar] [CrossRef] [Green Version]
- Makita, S.; Onoda, T.; Ohsawa, M.; Tanaka, F.; Segawa, T.; Takahashi, T.; Satoh, K.; Itai, K.; Tanno, K.; Sakata, K.; et al. Influence of mild-to-moderate alcohol consumption on cardiovascular diseases in men from the general population-ClinicalKey. Atherosclerosis 2012, 224, 222–227. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S0021915012004492?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0021915012004492%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F (accessed on 13 November 2019).
- Leong, D.P.; Smyth, A.; Teo, K.K.; McKee, M.; Rangarajan, S.; Pais, P.; Liu, L.; Anand, S.S.; Yusuf, S. INTERHEART Investigators Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case-control study. Circulation 2014, 130, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.; Kim, S.; Kang, H. Lifestyle Risk Factors and All-Cause and Cardiovascular Disease Mortality: Data from the Korean Longitudinal Study of Aging. Int. J. Environ. Res. Public Health 2019, 16, 3040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krokstad, S.; Ding, D.; Grunseit, A.C.; Sund, E.R.; Holmen, T.L.; Rangul, V.; Bauman, A. Multiple lifestyle behaviours and mortality, findings from a large population-based Norwegian cohort study-The HUNT Study. BMC Public Health 2017, 17, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.; Rogers, K.; van der Ploeg, H.; Stamatakis, E.; Bauman, A.E. Traditional and emerging lifestyle risk behaviors and all-cause mortality in middle-aged and older adults: Evidence from a large population-based Australian cohort. PLoS Med. 2015, 12, e1001917. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Suades, R.; Vilella-Figuerola, A.; Crespo, J.; Vilahur, G.; Escate, R.; Padro, T.; Chiva-Blanch, G. Liquid Biopsies: microvesicles in cardiovascular disease. Antioxid. Redox Signal. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stote, K.S.; Tracy, R.P.; Taylor, P.R.; Baer, D.J. The effect of moderate alcohol consumption on biomarkers of inflammation and hemostatic factors in postmenopausal women. Eur. J. Clin. Nutr. 2016, 70, 470–474. [Google Scholar] [CrossRef]
- Howard, R.; Scheiner, A.; Kanetsky, P.A.; Egan, K.M. Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann. Epidemiol. 2019, 38, 11–21. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar]
- Nova, E.; San Mauro-Martín, I.; Díaz-Prieto, L.E.; Marcos, A. Wine and beer within a moderate alcohol intake is associated with higher levels of HDL-c and adiponectin. Nutr. Res. 2019, 63, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antúnez, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: A randomised cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 46–53. [Google Scholar] [CrossRef] [PubMed]
- So-Armah, K.A.; Cheng, D.M.; Freiberg, M.S.; Gnatienko, N.; Patts, G.; Ma, Y.; White, L.; Blokhina, E.; Lioznov, D.; Doyle, M.F.; et al. Association between alcohol use and inflammatory biomarkers over time among younger adults with HIV—The Russia ARCH Observational Study. PLoS ONE 2019, 14, e0219710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørkhaug, S.T.; Neupane, S.P.; Bramness, J.G.; Aanes, H.; Skar, V.; Medhus, A.W.; Valeur, J. Plasma cytokine levels in patients with chronic alcohol overconsumption: relations to gut microbiota markers and clinical correlates. Alcohol 2019, in press. [Google Scholar]
- Kuprys, P.V.; Tsukamoto, H.; Gao, B.; Jia, L.; McGowan, J.; Coopersmith, C.M.; Moreno, M.C.; Hulsebus, H.; Meena, A.S.; Souza-Smith, F.M.; et al. Summary of the 2018 alcohol and immunology research interest group (AIRIG) meeting-ClinicalKey. Alcohol 2019, 77, 11–18. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S0741832918302076?scrollTo=%23hl0000358 (accessed on 19 November 2019). [CrossRef] [Green Version]
- Lawlor, D.A.; Nordestgaard, B.G.; Benn, M.; Zuccolo, L.; Tybjaerg-Hansen, A.; Davey Smith, G. Exploring causal associations between alcohol and coronary heart disease risk factors: findings from a Mendelian randomization study in the Copenhagen General Population Study. Eur. Heart J. 2013, 34, 2519–2528. [Google Scholar] [CrossRef] [Green Version]
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M.; Stampfer, M.J. Moderate alcohol intake and lower risk of coronary heart disease: Meta-analysis of effects on lipids and haemostatic factors. Br. Med. J. 1999, 319, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Vaughan, D.E.; Stampfer, M.J.; Glynn, R.J.; Hennekens, C.H. Association of moderate alcohol consumption and plasma concentration of endogenous tissue-type plasminogen activator. JAMA J. Am. Med. Assoc. 1994, 272, 929–933. [Google Scholar] [CrossRef]
- Smith, S.; Fair, K.; Goodman, A.; Watson, J.; Dodgion, C.; Schreiber, M. Consumption of alcohol leads to platelet inhibition in men. Am. J. Surg. 2019, 217, 868–872. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Massaro, J.M.; Ault, K.A.; Mittleman, M.A.; Sutherland, P.A.; Lipinska, I.; Levy, D.; D’Agostino, R.B.; Tofler, G.H. Alcohol consumption and platelet activation and aggregation among women and men: The Framingham Offspring Study. Alcohol. Clin. Exp. Res. 2005, 29, 1906–1912. [Google Scholar] [CrossRef]
- Wakabayashi, I. Platelet count in men with a habit of alcohol drinking. Platelets 2019, 1–3, in press. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Franchini, M. Alcohol consumption and venous thromboembolism: friend or foe? Intern. Emerg. Med. 2015, 10, 907–913. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.; Silva, E.R.; Foster, D.; McGee Harper, M.; Seidman, C.E.; Smith, J.D.; Breslow, J.L.; Brinton, E.A. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation 2000, 102, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Piano, M.R. Alcohol’s effects on the cardiovascular system. Alcohol Res. 2017, 38, 219–241. [Google Scholar]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Ben-Aicha, S.; Escate, R.; Casaní, L.; Padró, T.; Peña, E.; Arderiu, G.; Mendieta, G.; Badimón, L.; Vilahur, G. High-density lipoprotein remodelled in hypercholesterolaemic blood induce epigenetically driven down-regulation of endothelial HIF-1α expression in a preclinical animal model. Cardiovasc. Res. 2019, in press. [Google Scholar] [CrossRef] [Green Version]
- Li, X.H.; Yu, F.F.; Zhou, Y.H.; He, J. Association between alcohol consumption and the risk of incident type 2 diabetes: A systematic review and dose-response meta-analysis1. Am. J. Clin. Nutr. 2016, 103, 818–829. [Google Scholar] [CrossRef] [Green Version]
- Joosten, M.M.; Grobbee, D.E.; Van Der A, D.L.; Verschuren, W.M.M.; Hendriks, H.F.J.; Beulens, J.W.J. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. Am. J. Clin. Nutr. 2010, 91, 1777–1783. [Google Scholar] [CrossRef]
- Strelitz, J.; Ahern, A.L.; Long, G.H.; Boothby, C.E.; Wareham, N.J.; Griffin, S.J. Changes in behaviors after diagnosis of type 2 diabetes and 10-year incidence of cardiovascular disease and mortality. Cardiovasc. Diabetol. 2019, 18, 98. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, S.; Zhang, D.; He, S.; Lu, Y.; Gupta, A.; Spatz, E.S.; Lu, J.; Huang, C.; Herrin, J.; Liu, S.; et al. Prevalence, Awareness, and Treatment of Isolated Diastolic Hypertension: Insights from the China PEACE Million Persons Project. J. Am. Heart Assoc. 2019, 8, e012954. [Google Scholar] [CrossRef] [Green Version]
- Briasoulis, A.; Agarwal, V.; Messerli, F.H. Alcohol consumption and the risk of hypertension in men and women: A systematic review and meta-analysis. J. Clin. Hypertens. 2012, 14, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Jing, L.; Tian, Y.; Lin, M.; Du, Z.; Yan, H.; Ren, G.; Dong, Y.; Sun, Q.; Liu, S. Urban-Rural disparities in status of hypertension in northeast China: A population-based study, 2017–2019. Clin. Epidemiol. 2019, 11, 801–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2019, in press. [Google Scholar]
- Blomster, J.I.; Zoungas, S.; Chalmers, J.; Li, Q.; Chow, C.K.; Woodward, M.; Mancia, G.; Poulter, N.; Williams, B.; Harrap, S.; et al. The relationship between alcohol consumption and vascular complications and mortality in individuals with type 2 diabetes. Diabetes Care 2014, 37, 1353–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Castelnuovo, A.; Costanzo, S.; Bonaccio, M.; Rago, L.; De Curtis, A.; Persichillo, M.; Bracone, F.; Olivieri, M.; Cerletti, C.; Donati, M.B.; et al. Moderate alcohol consumption is associated with lower risk for heart failure but not atrial fibrillation. JACC Heart Fail. 2017, 5, 837–844. [Google Scholar] [CrossRef]
- Nishimura, M.; Bhatia, H.; Ma, J.; Dickson, S.D.; Alshawabkeh, L.; Adler, E.; Maisel, A.; Criqui, M.H.; Greenberg, B.; Thomas, I.C. The impact of substance abuse on heart failure hospitalizations-Clinicalkey. Am. J. Med. 2019, in press. [Google Scholar] [CrossRef]
- Costanzo, S.; Mukamal, K.J.; Di Castelnuovo, A.; Bonaccio, M.; Olivieri, M.; Persichillo, M.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; et al. Alcohol consumption and hospitalization burden in an adult Italian population: prospective results from the Moli-sani study. Addiction 2019, 114, 636–650. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Alcohol consumption and risk of heart failure: A dose-response meta-analysis of prospective studies. Eur. J. Heart Fail. 2015, 17, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979. [Google Scholar] [CrossRef]
- Goncalves, A.; Claggett, B.; Jhund, P.S.; Rosamond, W.; Deswal, A.; Aguilar, D.; Shah, A.M.; Cheng, S.; Solomon, S.D. Alcohol consumption and risk of heart failure: The Atherosclerosis Risk in Communities Study. Eur. Heart J. 2015, 36, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Lazo, M.; Chen, Y.; McEvoy, J.W.; Ndumele, C.; Konety, S.; Ballantyne, C.M.; Sharrett, A.R.; Selvin, E. Alcohol consumption and cardiac biomarkers: The atherosclerosis risk in communities (ARIC) study. Clin. Chem. 2016, 62, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef]
- Manthey, J.; Rehm, J. Mortality from alcoholic cardiomyopathy: Exploring the gap between estimated and civil registry data. J. Clin. Med. 2019, 8, 1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbano-Marquez, A.; Estruch, R.; Navarro-Lopez, F.; Grau, J.M.; Mont, L.; Rubin, E. The effects of alcoholism on skeletal and cardiac muscle. N. Engl. J. Med. 1989, 320, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; Teo, K.K.; Rangarajan, S.; O’Donnell, M.; Zhang, X.; Rana, P.; Leong, D.P.; Dagenais, G.; Seron, P.; Rosengren, A.; et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: A prospective cohort study. Lancet 2015, 386, 1945–1954. [Google Scholar] [CrossRef]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Naimi, T.S.; Stadtmueller, L.A.; Chikritzhs, T.; Stockwell, T.; Zhao, J.; Britton, A.; Saitz, R.; Sherk, A. Alcohol, age, and mortality: Estimating selection bias due to premature death. J. Stud. Alcohol Drugs 2019, 80, 63–68. [Google Scholar] [CrossRef]
- Roerecke, M.; Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: A narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014, 12, 182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Qin, Y.-Y.; Chen, Q.; Jiang, H.; Chen, X.-Z.; Xu, C.-L.; Mao, P.-J.; He, J.; Zhou, Y.-H. Alcohol intake and risk of stroke: a dose-response meta-analysis of prospective studies. Int. J. Cardiol. 2014, 174, 669–677. [Google Scholar] [CrossRef]
- Costa, P.; Grassi, M.; Iacoviello, L.; Zedde, M.; Marcheselli, S.; Silvestrelli, G.; DeLodovici, M.L.; Sessa, M.; Zini, A.; Paciaroni, M.; et al. Alcohol intake and the risk of intracerebral hemorrhage in the elderly: The MUCH-Italy. Neurology 2018, 91, e227–e235. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wallin, A.; Wolk, A.; Markus, H.S. Differing association of alcohol consumption with different stroke types: A systematic review and meta-analysis. BMC Med. 2016, 14, 178. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, J.F.; Ribeiro, A.L.P.; Santana, P.C.; Araújo, L.F.; Barreto, S.M. Relation of thoracic aortic and coronary artery calcium to cardiovascular risk factors (from The Brazilian Longitudinal Study of Adult Health [ELSA-Brazil])-ClinicalKey. Am. J. Cardiol. 2019, 124, 1655–1661. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S0002914919309956?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0002914919309956%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F (accessed on 29 October 2019). [CrossRef] [PubMed]
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol consumption and mortality in patients with cardiovascular disease: A Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 1339–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knott, C.S.; Coombs, N.; Stamatakis, E.; Biddulph, J.P. All cause mortality and the case for age specific alcohol consumption guidelines: Pooled analyses of up to 10 population based cohorts. BMJ 2015, 350, h384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockwell, T.; Zhao, J.; Panwar, S.; Roemer, A.; Naimi, T.; Chikritzhs, T. Do “Moderate” Drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J. Stud. Alcohol Drugs 2016, 77, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Xi, B.; Veeranki, S.P.; Zhao, M.; Ma, C.; Yan, Y.; Mi, J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults-ClinicalKey. J. Am. Coll. Cardiol. 2017, 70, 913–922. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S0735109717379986?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0735109717379986%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F (accessed on 8 November 2019). [CrossRef]
- Pai, J.K.; Mukamal, K.J.; Rimm, E.B. Long-term alcohol consumption in relation to all-cause and cardiovascular mortality among survivors of myocardial infarction: The Health Professionals Follow-up Study. Eur. Heart J. 2012, 33, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, J.H.; Bybee, K.A.; Lavie, C.J. Alcohol and cardiovascular health: The razor-sharp double-edged sword. J. Am. Coll. Cardiol. 2007, 50, 1009–1014. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, S.; de Gaetano, G.; Di Castelnuovo, A.; Djoussé, L.; Poli, A.; van Velden, D.P. Moderate alcohol consumption and lower total mortality risk: Justified doubts or established facts? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1003–1008. [Google Scholar] [CrossRef]
- Toma, A.; Paré, G.; Leong, D.P. Alcohol and cardiovascular disease: How much is too much? Curr. Atheroscler. Rep. 2017, 19, 13. [Google Scholar] [CrossRef]
- Sipilä, P.; Rose, R.J.; Kaprio, J. Drinking and mortality: long-term follow-up of drinking-discordant twin pairs. Addiction 2016, 111, 245–254. [Google Scholar] [CrossRef]
- Glymour, M.M. Alcohol and cardiovascular disease: New research tools will help us untangle this enigmatic association, eventually. BMJ 2014, 349, g4334. [Google Scholar] [CrossRef] [PubMed]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Badimon, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial epigenetics impact of polyphenols and phytochemicals in cancer prevention and therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronbaek, M.; Becker, U.; Johansen, D.; Gottschau, A.; Schnohr, P.; Hein, H.O.; Jensen, G.; Sorensen, T.I.A. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000, 133, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Woodward, M.; Doering, A.; Helbecque, N.; Loewel, H.; Amouyel, P.; Lowe, G.; Koenig, W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille). Eur. Heart J. 2004, 25, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- Malarcher, A.M.; Giles, W.H.; Croft, J.B.; Wozniak, M.A.; Wityk, R.J.; Stolley, P.D.; Stern, B.J.; Sloan, M.A.; Sherwin, R.; Price, T.R.; et al. Alcohol intake, type of beverage, and the risk of cerebral infarction in young women. Stroke 2001, 32, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [Green Version]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 36–45. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepoli, M.F.; Abreu, A.; Albus, C.; Ambrosetti, M.; Brotons, C.; Catapano, A.L.; Corra, U.; Cosyns, B.; Deaton, C.; Graham, I.; et al. Update on cardiovascular prevention in clinical practice: A position paper of the European Association of Preventive Cardiology of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2019, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, M.A.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; Michos, E.D.; Miedema, M.D.; Muñoz, D.; Smith, S.C.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar]
- Van de Luitgaarden, I.A.T.; Beulens, J.W.J.; Schrieks, I.C.; Kieneker, L.M.; Touw, D.J.; van Ballegooijen, A.J.; van Oort, S.; Grobbee, D.E.; Bakker, S.J.L. Urinary ethyl glucuronide can be used as a biomarker of habitual alcohol consumption in the general population. J. Nutr. 2019, 149, 2199–2205. [Google Scholar] [CrossRef]
- Kloner, R.A.; Rezkalla, S.H. To drink or not to drink? That is the question. Circulation 2007, 116, 1306–1317. [Google Scholar] [CrossRef]
- Abat, C.; Roussel, Y.; Chaudet, H.; Raoult, D. Alcohol and the global burden of disease. Lancet 2019, 393, 2390–2391. [Google Scholar] [CrossRef] [Green Version]
| Drinking Level | Number of Drinks 1 | Amount of Alcohol |
|---|---|---|
| Low-risk consumption | ≤3 drinks on a single day for women | ≤32 g/single day (occasional) |
| ≤7 drinks/week for women | ≤14 g/day (regular) | |
| ≤4 drinks on a single day for men | ≤46 g/single day (occasional) | |
| ≤14 drinks/week for men | ≤28 g/day (regular) | |
| Moderate | ≤1 drink/day for women | ≤14 g/day (regular) |
| ≤2 drink/day for men | ≤28 g/day (regular) | |
| Binge | ≥4 drinks in 2 h for women | 56 g in one occasion |
| ≥5 drinks in 2 h for men | 70 g in one occasion | |
| Heavy | ≥5 binge drinking days in a month | ≥280 g in a month |
| Cardiovascular Parameter | Low/Moderate Alcohol Consumption | Heavy/Binge Drinking |
|---|---|---|
| Intermediate biomarkers | ||
| Inflammation | + 1 | − |
| Oxidation | ± 1 | − |
| Thrombosis | + | ± |
| Classical risk factors | ||
| Lipid profile | + | − |
| Glucose metabolism | + | ± |
| Blood pressure | − | − |
| Major adverse cardiovascular events | ||
| Acute myocardial infarction | + | ± |
| Stroke | + | − |
| Cardiovascular mortality | + | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiva-Blanch, G.; Badimon, L. Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies. Nutrients 2020, 12, 108. https://doi.org/10.3390/nu12010108
Chiva-Blanch G, Badimon L. Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies. Nutrients. 2020; 12(1):108. https://doi.org/10.3390/nu12010108
Chicago/Turabian StyleChiva-Blanch, Gemma, and Lina Badimon. 2020. "Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies" Nutrients 12, no. 1: 108. https://doi.org/10.3390/nu12010108
APA StyleChiva-Blanch, G., & Badimon, L. (2020). Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies. Nutrients, 12(1), 108. https://doi.org/10.3390/nu12010108