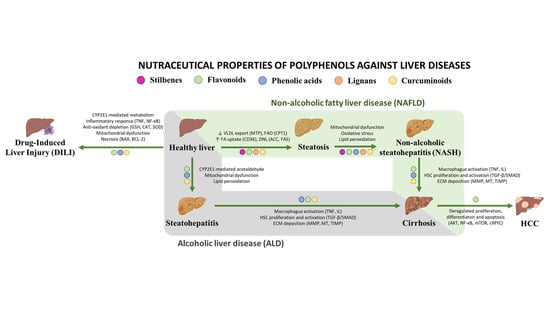
Nutraceutical Properties of Polyphenols against Liver Diseases
Abstract
:1. Introduction
2. Polyphenols and Their Nutraceutical Value
2.1. Stilbenes
2.2. Flavonoids
2.3. Phenolic Acids
2.4. Lignans
2.5. Curcuminoids
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasutake, K.; Kohjima, M.; Kotoh, K.; Nakashima, M.; Nakamuta, M.; Enjoji, M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1756–1767. [Google Scholar] [CrossRef]
- Micha, R.; Shulkin, M.L.; Peñalvo, J.L.; Khatibzadeh, S.; Singh, G.M.; Rao, M.; Fahimi, S.; Powles, J.; Mozaffarian, D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE 2017, 12, e0175149. [Google Scholar] [CrossRef]
- Tarasenko, T.N.; McGuire, P.J. The liver is a metabolic and immunologic organ: A reconsideration of metabolic decompensation due to infection in inborn errors of metabolism (IEM). Mol. Genet. Metab. 2017, 121, 283–288. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Mishra, A.; Younossi, Z.M. Epidemiology and Natural History of Non-alcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2012, 2, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.A.; Lymp, J.F.; St. Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Day, C.P. From fat to inflammation. Gastroenterology 2006, 130, 207–210. [Google Scholar] [CrossRef]
- Noureddin, M.; Rinella, M.E. Nonalcoholic Fatty Liver Disease, Diabetes, Obesity, and Hepatocellular Carcinoma. Clin. Liver Dis. 2015, 19, 361–379. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Lisby, A.; Ma, C.; Lo, N.; Ehmer, U.; Hayer, K.E.; Furth, E.E.; Viatour, P. Promotion of growth factor signaling as a critical function of β-catenin during HCC progression. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- David, S.; Hamilton, J.P. Drug-induced Liver Injury. US Gastroenterol. Hepatol. 2010, 6, 73–80. [Google Scholar]
- He, Y.; Jin, L.; Wang, J.; Yan, Z.; Chen, T.; Zhao, Y. Mechanisms of fibrosis in acute liver failure. Liver Int. 2015, 35, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Giordano, C.; Rivas, J.; Zervos, X. An Update on Treatment of Drug-Induced Liver Injury. J. Clin. Transl. Hepatol. 2014, 2, 74–79. [Google Scholar]
- Bell, L.N.; Chalasani, N. Epidemiology of idiosyncratic drug-induced liver injury. Semin. Liver Dis. 2009, 29, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Nelson, L.J.; Gómez Del Moral, M.; Martínez-Naves, E.; Cubero, F.J. Dissecting the molecular pathophysiology of drug-induced liver injury. World J. Gastroenterol. 2018, 24, 1373–1385. [Google Scholar] [CrossRef]
- Bajt, M.L.; Ramachandran, A.; Yan, H.-M.; Lebofsky, M.; Farhood, A.; Lemasters, J.J.; Jaeschke, H. Apoptosis-Inducing Factor Modulates Mitochondrial Oxidant Stress in Acetaminophen Hepatotoxicity. Toxicol. Sci. 2011, 122, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Jaeschke, H.; Duan, L.; Akakpo, J.Y.; Farhood, A.; Ramachandran, A. The role of apoptosis in acetaminophen hepatotoxicity. Food Chem. Toxicol. 2018, 118, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Quan, X.-B.; Zeng, W.-J.; Yang, X.-O.; Wang, M.-J. Mechanism of Hepatocyte Apoptosis. J. Cell Death 2016, 9, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basra, S.; Anand, B.S. Definition, epidemiology and magnitude of alcoholic hepatitis. World J. Hepatol. 2011, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 2017, 38, 147–161. [Google Scholar]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Petrides, J.; Collins, P.; Kowalski, A.; Sepede, J.; Vermeulen, M. Lifestyle Changes for Disease Prevention. Prim. Care 2019, 46, 1–12. [Google Scholar] [CrossRef]
- Al-Dashti, Y.A.; Holt, R.R.; Stebbins, C.L.; Keen, C.L.; Hackman, R.M. Dietary Flavanols: A Review of Select Effects on Vascular Function, Blood Pressure, and Exercise Performance. J. Am. Coll. Nutr. 2018, 37, 553–567. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Nguyen, N.U.; Stamper, B.D. Polyphenols reported to shift APAP-induced changes in MAPK signaling and toxicity outcomes. Chem. Biol. Interact. 2017, 277, 129–136. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Kang, M.-G.; Cha, H.Y.; Kim, Y.M.; Lim, Y.; Yang, S.J. Effects of Piceatannol and Resveratrol on Sirtuins and Hepatic Inflammation in High-Fat Diet-Fed Mice. J. Med. Food 2019, 22, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Bishayee, A.; Darvesh, A.S.; Politis, T.; McGory, R. Resveratrol and liver disease: Frombench to bedside and community. Liver Int. 2010, 30, 1103–1114. [Google Scholar] [CrossRef]
- Peiyuan, H.; Zhiping, H.; Chengjun, S.; Chunqing, W.; Bingqing, L.; Imam, M.U. Resveratrol Ameliorates Experimental Alcoholic Liver Disease by Modulating Oxidative Stress. Evidence-Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.M.O.; Paraíso, A.F.; de Oliveira, M.V.M.; Martins, A.M.E.; Neto, J.F.; Guimarães, A.L.S.; de Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the beta-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis 2010, 31, 1272–1278. [Google Scholar] [CrossRef]
- Aguirre, L.; Palacios-ortega, S.; Fernández-Quintela, A.; Hijona, E.; Bujanda, L.; Portillo, M.P. Pterostilbene reduces liver steatosis and modifies hepatic fatty acid profile in obese rats. Nutrients 2019, 11, 961. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Zorita, S.; Milton-Laskibar, I.; Aguirre, L.; Fernandez-Quintela, A.; Xiao, J.; Portillo, M.P. Effects of Pterostilbene on Diabetes, Liver Steatosis and Serum Lipids. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubiete-Franco, I.; Garcia-Rodriguez, J.L.; Martinez-Una, M.; Martinez-Lopez, N.; Woodhoo, A.; Juan, V.G.-D.; Beraza, N.; Lage-Medina, S.; Andrade, F.; Fernandez, M.L.; et al. Methionine and S-adenosylmethionine levels are critical regulators of PP2A activity modulating lipophagy during steatosis. J. Hepatol. 2016, 64, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef]
- Watson, R.R.; Schönlau, F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: A review. Minerva Cardioangiol. 2015, 63, 1–12. [Google Scholar]
- Parra-Vargas, M.; Sandoval-Rodriguez, A.; Rodriguez-Echevarria, R.; Dominguez-Rosales, J.A.; Santos-Garcia, A.; Armendariz-Borunda, J. Delphinidin ameliorates hepatic triglyceride accumulation in human HepG2 cells, but not in diet-induced obese mice. Nutrients 2018, 10, 1060. [Google Scholar] [CrossRef] [Green Version]
- Domitrovic, R.; Jakovac, H. Antifibrotic activity of anthocyanidin delphinidin in carbon tetrachloride-induced hepatotoxicity in mice. Toxicology 2010, 272, 1–10. [Google Scholar] [CrossRef]
- Lee, W.; Lee, Y.; Kim, J.; Bae, J.-S. Protective Effects of Pelargonidin on Lipopolysaccharide-induced Hepatic Failure. Nat. Prod. Commun. 2018, 13, 1934578X1801300114. [Google Scholar] [CrossRef] [Green Version]
- Andersen, Ø; Jordheim, M. Basic Anthocyanin Chemistry and Dietary Source. In Anthocyanins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2013; pp. 13–90. ISBN 9781439894712. [Google Scholar]
- Park, S.; Kang, S.; Jeong, D.-Y.Y.; Jeong, S.-Y.Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 455. [Google Scholar] [CrossRef]
- Bendia, E.; Benedetti, A.; Baroni, G.S.; Candelaresi, C.; Macarri, G.; Trozzi, L.; Di Sario, A. Effect of cyanidin 3-O-beta-glucopyranoside on hepatic stellate cell proliferation and collagen synthesis induced by oxidative stress. Dig. Liver Dis. 2005, 37, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-beta-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wang, S.; Ye, M.; Ling, W.; Yang, L. Cyanidin-3-O-β-glucoside protects against liver fibrosis induced by alcohol via regulating energy homeostasis and AMPK/autophagy signaling pathway. J. Funct. Foods 2017, 37, 16–24. [Google Scholar] [CrossRef]
- Tulio, A.Z.J.; Reese, R.N.; Wyzgoski, F.J.; Rinaldi, P.L.; Fu, R.; Scheerens, J.C.; Miller, A.R. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J. Agric. Food Chem. 2008, 56, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, J.; Tian, J.; Si, X.; Jiao, X.; Zhang, W.; Gong, E.; Li, B. Blueberry Malvidin-3-galactoside Suppresses Hepatocellular Carcinoma by Regulating Apoptosis, Proliferation, and Metastasis Pathways In Vivo and In Vitro. J. Agric. Food Chem. 2019, 67, 625–636. [Google Scholar] [CrossRef]
- Jaramillo Flores, M.E. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dower, J.I.; Geleijnse, J.M.; Kroon, P.A.; Philo, M.; Mensink, M.; Kromhout, D.; Hollman, P.C.H. Does epicatechin contribute to the acute vascular function effects of dark chocolate? A randomized, crossover study. Mol. Nutr. Food Res. 2016, 60, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xu, N.; Zhao, W.; Su, J.; Liang, M.; Xie, Z.; Wu, X.; Li, Q. (-)-Epicatechin regulates blood lipids and attenuates hepatic steatosis in rats fed high-fat diet. Mol. Nutr. Food Res. 2017, 61, 1700303. [Google Scholar] [CrossRef]
- Huang, Z.; Jing, X.; Sheng, Y.; Zhang, J.; Hao, Z.; Wang, Z.; Ji, L. (-)-Epicatechin attenuates hepatic sinusoidal obstruction syndrome by inhibiting liver oxidative and inflammatory injury. Redox Biol. 2019, 22, 101117. [Google Scholar] [CrossRef]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Q.; Liu, L.; Hu, Y.Y.; Feng, Q. Potential Biological Effects of (-)-Epigallocatechin-3-gallate on the Treatment of Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2018, 62, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.T.; Li, C.C.; Chang, C.H. Epigallocatechin-3-gallate reduces hepatic oxidative stress and lowers cyp-mediated bioactivation and toxicity of acetaminophen in rats. Nutrients 2019, 11, 1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimonte, S.; Albino, V.; Piccirillo, M.; Nasto, A.; Molino, C.; Palaia, R.; Cascella, M. Epigallocatechin-3-gallate in the prevention and treatment of hepatocellular carcinoma: Experimental findings and translational perspectives. Drug Des. Devel. Ther. 2019, 13, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.F.; Dai, M.G. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Su, B.; Fan, S.; Fei, H.; Zhao, W. Protective effect of oligomeric proanthocyanidins against alcohol-induced liver steatosis and injury in mice. Biochem. Biophys. Res. Commun. 2015, 458, 757–762. [Google Scholar] [CrossRef]
- Miltonprabu, S.; Nazimabashir; Manoharan, V. Hepatoprotective effect of grape seed proanthocyanidins on Cadmium-induced hepatic injury in rats: Possible involvement of mitochondrial dysfunction, inflammation and apoptosis. Toxicol. Rep. 2016, 3, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H. Procyanidin content and variation in some commonly consumed foods. J. Nutr. 2000, 130, 2086S–2092S. [Google Scholar] [CrossRef]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxid. Med. Cell. Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef]
- Guedon, D.J.; Pasquier, B.P. Analysis and Distribution of Flavonoid Glycosides and Rosmarinic Acid in 40 Mentha x piperita Clones. J. Agric. Food Chem. 1994, 42, 679–684. [Google Scholar] [CrossRef]
- Ooghe, W.C.; Detavernier, C.M. Detection of the Addition of Citrus reticulata and Hybrids to Citrus sinensis by Flavonoids. J. Agric. Food Chem. 1997, 45, 1633–1637. [Google Scholar] [CrossRef]
- Çetin, A.; Çiftçi, O.; Otlu, A. Protective effect of hesperidin on oxidative and histological liver damage following carbon tetrachloride administration in Wistar rats. Arch. Med. Sci. 2016, 12, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Karthikeyan, A.; Karthika, P.; Rathinam, A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol. Rep. 2015, 2, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.-Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compos. Anal. Off. Publ. United Nations Univ. Int. Netw. Food Data Syst. 2007, 20, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, O.M.; Fahim, H.I.; Ahmed, H.Y.; Al-Muzafar, H.M.; Ahmed, R.R.; Amin, K.A.; El-Nahass, E.S.; Abdelazeem, W.H. The preventive effects and the mechanisms of action of navel orange peel hydroethanolic extract, naringin, and naringenin in N-Acetyl-p-aminophenol-induced liver injury in wistar rats. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010, 6, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, X.; Li, H.; Yan, Y.; Hong, X.; Lin, Z. Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21. Int. J. Immunopathol. Pharmacol. 2018, 32. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Huang, S.; Huang, Q.; Ming, Z.; Wang, M.; Li, R.; Zhao, Y. Kaempferol attenuates liver fibrosis by inhibiting activin receptor–like kinase 5. J. Cell. Mol. Med. 2019, 23, 6403–6410. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.F.; Le, G.W.; Wang, P.; Qiu, Y.Y.; Jiang, Y.Y.; Tang, X. Regressive effect of myricetin on hepatic steatosis in mice fed a high-fat diet. Nutrients 2016, 8, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Chen, J.; Yu, X.; Xu, S.; Li, D.; Zheng, Q.; Yin, Y. Myricetin Suppresses the Propagation of Hepatocellular Carcinoma via Down-Regulating Expression of YAP. Cells 2019, 8, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganbold, M.; Owada, Y.; Ozawa, Y.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Zheng, Y.W.; Ohkohchi, N.; Isoda, H. Isorhamnetin Alleviates Steatosis and Fibrosis in Mice with Nonalcoholic Steatohepatitis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, K.; Ohmuma, M. Effects of isorhamnetin, rhamnetin, and quercetin on the concentrations of cholesterol and lipoperoxide in the serum and liver and on the blood and liver antioxidative enzyme activities of rats. Biosci. Biotechnol. Biochem. 1995, 59, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Kim, S.C.; Kim, K.M.; Jang, C.H.; Cho, S.S.; Kim, S.J.; Ku, S.K.; Cho, I.J.; Ki, S.H. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-beta/Smad signaling and relieving oxidative stress. Eur. J. Pharmacol. 2016, 783, 92–102. [Google Scholar] [CrossRef]
- Huang, H.; Chen, A.Y.; Ye, X.; Guan, R.; Rankin, G.O.; Chen, Y.C. Galangin, a Flavonoid from Lesser Galangal, Induced Apoptosis via p53-Dependent Pathway in Ovarian Cancer Cells. Molecules 2020, 25, 1579. [Google Scholar] [CrossRef] [Green Version]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.M.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Chen, X.; Wu, J.; Lin, B.; Zhang, H.; Lan, L.; Luo, H. Galangin inhibits proliferation of hepatocellular carcinoma cells by inducing endoplasmic reticulum stress. Food Chem. Toxicol. 2013, 62, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Liu, J.-C.; Zhou, R.-J.; Zhao, X.; Liu, M.; Ye, H.; Xie, M.-L. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARalpha-mediated lipogenic gene expression. Chem. Biol. Interact. 2017, 275, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Tsaroucha, A.K.; Tsiaousidou, A.; Ouzounidis, N.; Tsalkidou, E.; Lambropoulou, M.; Giakoustidis, D.; Chatzaki, E.; Simopoulos, C. Intraperitoneal administration of apigenin in liver ischemia/reperfusion injury protective effects. Saudi J. Gastroenterol. 2016, 22, 415–422. [Google Scholar] [PubMed]
- Jung, U.J.; Cho, Y.-Y.; Choi, M.-S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef] [Green Version]
- Balam, F.H.; Ahmadi, Z.S.; Ghorbani, A. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): A systematic review. Heliyon 2020, 6, e03557. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.-S.; Juvekar, A.R. Chrysin ameliorates nonalcoholic fatty liver disease in rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 1617–1628. [Google Scholar] [CrossRef]
- Balta, C.; Ciceu, A.; Herman, H.; Rosu, M.; Boldura, O.M.; Hermenean, A. Dose-dependent antifibrotic effect of chrysin on regression of liver fibrosis: The role in extracellular matrix remodeling. Dose-Response 2018, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Tai, M.; Zhang, J.; Song, S.; Miao, R.; Liu, S.; Pang, Q.; Wu, Q.; Liu, C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int. Immunopharmacol. 2015, 27, 164–170. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, X.; Yang, D.; Lu, J.; Liu, B.; Baiyun, R.; Zhang, Z. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget 2017, 8, 40982–40993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Zhang, Y.; Liu, C.; Xu, D.; Zhang, R.; Cheng, Y.; Pan, Y.; Huang, C.; Chen, Y. Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. J. Nutr. 2014, 144, 1009–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, M.R.; Cummings, J.H.; Morton, M.S.; Steel, C.M.; Bolton-Smith, C.; Riches, A.C. A newly constructed and validated isoflavone database for the assessment of total genistein and daidzein intake. Br. J. Nutr. 2006, 95, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.; Chen, C.; Hu, Y.-Y.; Feng, Q. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). Biomed. Pharmacother. 2019, 117, 109047. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, H.; Zheng, Z.; Lu, R.; Jiang, Z. Genistein can ameliorate hepatic inflammatory reaction in nonalcoholic steatohepatitis rats. Biomed. Pharmacother. 2019, 111, 1290–1296. [Google Scholar] [CrossRef]
- Kuzu, N.; Metin, K.; Dagli, A.F.; Akdemir, F.; Orhan, C.; Yalniz, M.; Ozercan, I.H.; Sahin, K.; Bahcecioglu, I.H. Protective role of genistein in acute liver damage induced by carbon tetrachloride. Mediators Inflamm. 2007, 2007. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Odbayar, T.O.; Ide, T. A comparative analysis of genistein and daidzein in affecting lipid metabolism in rat liver. J. Clin. Biochem. Nutr. 2009, 44, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Park, J.-S.; Jung, J.-W.; Byun, K.-W.; Kang, K.-S.; Lee, Y.-S. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int. J. Obes. (Lond.) 2011, 35, 1019–1030. [Google Scholar] [CrossRef] [Green Version]
- Cheraghpour, M.; Imani, H.; Ommi, S.; Alavian, S.M.; Karimi-Shahrbabak, E.; Hedayati, M.; Yari, Z.; Hekmatdoost, A. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled, double-blind clinical trial. Phytother. Res. 2019, 33, 2118–2125. [Google Scholar] [CrossRef]
- Nahmias, Y.; Goldwasser, J.; Casali, M.; van Poll, D.; Wakita, T.; Chung, R.T.; Yarmush, M.L. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 2008, 47, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, O.; Fontanes, V.; Raychaudhuri, S.; Loo, R.; Loo, J.; Arumugaswami, V.; Sun, R.; Dasgupta, A.; French, S.W. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology 2009, 50, 1756–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amsterdam, Netherlands) 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms. Adv. Nutr. 2016, 7, 961–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018, 8, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Kapan, M.; Gumus, M.; Onder, A.; Firat, U.; Basarali, M.K.; Boyuk, A.; Aliosmanoglu, I.; Buyukbas, S. The effects of ellagic acid on the liver and remote organs’ oxidative stress and structure after hepatic ischemia reperfusion injury caused by pringle maneuver in rats. Bratisl. Lek. Listy 2012, 113, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Aslan, A.; Gok, O.; Erman, O.; Kuloglu, T. Ellagic acid impedes carbontetrachloride-induced liver damage in rats through suppression of NF-kB, Bcl-2 and regulating Nrf-2 and caspase pathway. Biomed. Pharmacother. 2018, 105, 662–669. [Google Scholar] [CrossRef]
- Setayesh, T.; Nersesyan, A.; Mišík, M.; Noorizadeh, R.; Haslinger, E.; Javaheri, T.; Lang, E.; Grusch, M.; Huber, W.; Haslberger, A.; et al. Gallic acid, a common dietary phenolic protects against high fat diet induced DNA damage. Eur. J. Nutr. 2019, 58, 2315–2326. [Google Scholar] [CrossRef] [Green Version]
- Rasool, M.K.; Sabina, E.P.; Ramya, S.R.; Preety, P.; Patel, S.; Mandal, N.; Mishra, P.P.; Samuel, J. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 2010, 62, 638–643. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Kurt, H.; Bayramoglu, A.; Gunes, H.V.; Degirmenci, İ.; Colak, S. Preventive role of gallic acid on hepatic ischemia and reperfusion injury in rats. Cytotechnology 2015, 67, 845–849. [Google Scholar] [CrossRef] [Green Version]
- El-Lakkany, N.M.; El-Maadawy, W.H.; Seif el-Din, S.H.; Saleh, S.; Safar, M.M.; Ezzat, S.M.; Mohamed, S.H.; Botros, S.S.; Demerdash, Z.; Hammam, O.A. Antifibrotic effects of gallic acid on hepatic stellate cells: In vitro and in vivo mechanistic study. J. Tradit. Complement. Med. 2019, 9, 45–53. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. (Amsterdam, Netherlands) 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. 2020, 27, 7910–7921. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Zuo, S.; Wu, R.M.; Deng, K.S.; Lu, S.; Zhu, J.J.; Zou, G.L.; Yang, J.; Cheng, M.L.; Zhao, X.K. Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway. Drug Des. Dev. Ther. 2018, 12, 4107–4115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2017, 15, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Pan, J.H.; Kim, S.H.; Lee, J.H.; Park, J.-W. Chlorogenic acid ameliorates alcohol-induced liver injuries through scavenging reactive oxygen species. Biochimie 2018, 150, 131–138. [Google Scholar] [CrossRef]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef]
- Jemai, H.; Mahmoudi, A.; Feryeni, A.; Fki, I.; Bouallagui, Z.; Choura, S.; Chamkha, M.; Sayadi, S. Hepatoprotective Effect of Oleuropein-Rich Extract from Olive Leaves against Cadmium-Induced Toxicity in Mice. BioMed Res. Int. 2020, 2020, 4398924. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.; Um, S.-J.; Yoon, S.K.; Park, T. Oleuropein attenuates hepatic steatosis induced by high-fat diet in mice. J. Hepatol. 2011, 54, 984–993. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [Green Version]
- Sirato-Yasumoto, S.; Katsuta, M.; Okuyama, Y.; Takahashi, Y.; Ide, T. Effect of Sesame Seeds Rich in Sesamin and Sesamolin on Fatty Acid Oxidation in Rat Liver. J. Agric. Food Chem. 2001, 49, 2647–2651. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Ashakumary, L.; Takahashi, Y.; Kushiro, M.; Fukuda, N.; Sugano, M. Sesamin, a sesame lignan, decreases fatty acid synthesis in rat liver accompanying the down-regulation of sterol regulatory element binding protein-1. Biochim. Biophys. Acta 2001, 1534, 1–13. [Google Scholar] [CrossRef]
- Frank, J.; Eliasson, C.; Leroy-Nivard, D.; Budek, A.; Lundh, T.; Vessby, B.; Aman, P.; Kamal-Eldin, A. Dietary secoisolariciresinol diglucoside and its oligomers with 3-hydroxy-3-methyl glutaric acid decrease vitamin E levels in rats. Br. J. Nutr. 2004, 92, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Felmlee, M.A.; Woo, G.; Simko, E.; Krol, E.S.; Muir, A.D.; Alcorn, J. Effects of the flaxseed lignans secoisolariciresinol diglucoside and its aglycone on serum and hepatic lipids in hyperlipidaemic rats. Br. J. Nutr. 2009, 102, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
| Polyphenol | Group/Subgroup | Pathology | Outcome |
|---|---|---|---|
| Resveratrol | Stilbenes | NAFLD, HCC, Hepatitis | Improved inflammatory profile in NAFLD [44]. |
| Hesperidin | Flavonoids/Flavanones | NASH | Ameliorated steatosis, hepatic enzymes and glycaemia [110]. |
| Naringenin | Flavonoids/Flavanones | Hepatitis C | Ameliorated phenotype [111]. |
| Quercetin | Flavonoids/Flavonols | Hepatitis C | Attenuated secretion of the virus [112] |
| Procyanidins | Procyanidins/Flavanols | NAFLD | Not finished |
| EGCG | Flavonoids/Flavanols | Cirrhosis-derived HCC | Not finished |
| Gallic acid | Phenolic acids/Hydroxibenzoic acids | NAFLD | Atherosclerosis reduction. |
| Chlorogenic acid | Phenolic acids/Hydroxicinnamic acids | NAFLD | Not published |
| Curcumin | Curcuminoids | NAFLD | Reduction in steatosis and body-mass index and improved serum profile [138] |
| Polyphenol | Group/Subgroup | Source | Liver Pathology | Molecular Targets |
|---|---|---|---|---|
| Resveratrol | Stilbenes | Coco, mulberries, peanuts, soy and grapes [34] | Steatosis/NASH | Glutathione, CYP2E1 [35,36] |
| Steatosis | SIRT1, ACC, PPARγ, SREBP-1 [37] | |||
| Pterostilbene | Stilbenes | Blueberries [38] | Steatosis | Glucokinase, Glucose-6-phosphatase [40] |
| Steatosis | CPT1, MTP, CD36 [39] | |||
| Piceatannol | Stilbenes | Grapes, passion fruit and peanut calluses [33] | Steatosis | AMPK, ACC, FAS and autophagy [42] |
| Delphinidin | Flavonoids/anthocyanins | Flowers, blueberry, Saskatoon berry, raspberry, strawberry, chokecherry, Maqui berry [45] | NASH/ALD | NF-κB, AP-1, COX-2 [46] |
| Steatosis | AMPK, FAS [47] | |||
| Fibrosis | Oxidative stress, MMP-9 and MT [48] | |||
| Pelargonidin | Flavonoids/Anthocyanins | Raspberries, blackberries, strawberries or plums [50] | NASH/ALD | TLR [49] |
| Cyanidin | Flavonoids/Anthocyanins | Red berries, grapes, bilberry, blackberry, blueberry, cherry, cranberry, elderberry, hawthorn, loganberry, açaai berry and raspberry [55] | Steatosis | CPT1, PPARα, FAS, SREBP-1 [51] |
| Fibrosis | Collagen I, ERK 1/2 [52] | |||
| NASH/Fibrosis | PKA, GSH [53] | |||
| ALD | AMPK [54] | |||
| Malvidin | Flavonoids/Anthocyanins | Red grapes, cranberries, blueberries and black rice [80] | Steatosis | CPT1, PPARα, FAS, SREBP-1 [51] |
| HCC | BAX, Caspase-3, Cyclin, PTEN, MMP-2/9 [56] | |||
| Epicatechin | Flavonoids/Flavanols | Dark chocolate and cocoa [58] | Steatosis | SREBP-1, FAS, LXR, SIRT [59] |
| DILI/ALD | Bile acid and lipid absorption [60] | |||
| Epigallocatechin/EGCG | Flavonoids/Flavanols | Green tea [61] | NASH | NF-κB [60] |
| Steatosis/NASH | AMPK, SREBP-1, FAS, ACC; CYP2E1, malonaldehyde, TNF, IL; TGF/SMAD [62] | |||
| Fibrosis | Collagen, αSMA, TIMP-2 [63] | |||
| DILI | CYP [64] | |||
| HCC | NF-κB, BCL2; cMYC, ERK1/2, DDR; MMP, COX-2 [65] | |||
| Procyanidins | Flavonoids/Flavanols | Chocolate, apples, red grapes and cranberries [69] | NASH/Fibrosis | CYP2E1. GSH, SOD [66] |
| ALD | SREBP-1, IL-6, TNF [67] | |||
| NASH/ALD/DILI | Mitochondrial dysfunction and apoptosis [68] | |||
| Hesperidin | Flavonoids/Flavanones | Citrus fruits and peppermint [71,72] | NASH/Fibrosis | GSH, CAT, SOD [73] |
| Steatosis/NASH | Lipoperoxidation [74] | |||
| Naringenin | Flavonoids/Flavanones | Mexican oregano [75] | DILI | Caspase-3, BAX, BCL [76] |
| Fibrosis | TGF-β, ECM deposition [77] | |||
| Quercetin | Flavonoids/Flavonols | Apples, berries, brassica vegetables, capers, grapes, onions, shallots, tea, tomatoes, seeds and nuts [78,79] | Fibrosis | NF-κB, TNF, IL-1β, IL-6, IL-8 [78] |
| ALD | GSH, IL-10, lipid peroxidation [79] | |||
| Kaempferol | Flavonoids/Flavonols | Tea, broccoli, apples, strawberries and beans [80] | Fibrosis | ALK5, SMAD 2/3 |
| HCC | PTEN, PI3K/AKT/mTOR [81] | |||
| ALD | CYP2E1 [82] | |||
| Myricetin | Flavonoids/Flavonols | Berries, honey, vegetables, teas and wines [84] | Steatosis/NASH | NRF-2, mitochondrial functionality, PPAR [85] |
| HCC | YAP [86] | |||
| Isorhamnetin | Flavonoids/Flavonols | Pears, onion, olive oil, grapes, tomato, Mexican Tarragon [80,89] | Steatosis/NASH/Fibrosis | FAS, TGF.β, HSC activation [87] |
| NASH | Lipoperoxidation [88] | |||
| Galangin | Flavonoids/Flavonols | Rizhome and propolis [90] | NASH/DILI | NRF-2, apoptosis [91] |
| HCC | NRF-2, HO-1 [92] | |||
| Apigenin | Flavonoids/Flavones | Parsley, broccoli, celery, onions, oranges, olives, cherries, tomatoes, chamomile, thyme, oregano, basil, tea [93] | ALD | CYP2E1, PPARα [94] |
| Steatosis | FAO, Tricarboxylic acid cycle, oxidative phosphorylation [96] | |||
| Chrysin | Flavonoids/Flavones | Honey and propolis [97] | Steatosis/NASH | TNF, IL-6, SREBP-1 [98] |
| Fibrosis | MMP, TIMP [99] | |||
| Luteolin | Flavonoids/Flavones | Celery, parsley, broccoli, onion, carrots, peppers, cabbages and apple [100] | DILI | GSH, TNF, NF-κB, IL-6, ER stress [101] |
| Fibrosis/DILI | NRF-2, NF-κB, P53 [102] | |||
| ALD | SREBP-1, AMPK [103] | |||
| Genistein | Flavonoids/Isoflanoids | Soybeans, nuts and legumes [104] | Steatosis | PPARα [105] |
| NASH | TLR4 [106] | |||
| Fibrosis | Lipoperoxidation, GSH [107] | |||
| Daidzein | Flavonoids/Isoflanoids | Soybeans, nuts and legumes [104] | Steatosis/NASH | FAO, TNF [109] |
| Ellagic acid | Phenolic acids/Hydroxibenzoic acids | Nuts, walnuts, berries, pomegranades or berries [114] | NASH/DILI/ALD | Oxidative stress [115] |
| IR | Oxidative stress [116] | |||
| Fibrosis | Caspase-3, BCL-2, NF-kB, NRF-2 [117] aslan | |||
| Gallic acid | Phenolic acids/Hydroxibenzoic acids | Blueberries, strawberries and mango [118] | Fibrosis | GSH, TGF-β [121] |
| DILI/ALD | TNF, lipoperoxidation [119] | |||
| IR | GSH and CAT [120] | |||
| Ferulic acid | Phenolic acids/Hydroxycinnamic acids | Rice, wheat, oats, grains, vegetables, pineapple, beans, coffee, artichoke, peanut, nuts [122] | DILI | NRF-2/HO-1 [123] |
| Fibrosis | TGF-β/SMAD [124] | |||
| Cholorogenic acid | Phenolic acids/Hydroxycinnamic acids | Coffee, beans, potato, apple and prunes [125] | Fibrosis | TNF, IL-6 and IL-1β [126] |
| ALD | ROS, TNF, TGF-β [127] | |||
| Oleuropein | Phenolc acids/Oleuropeunosides | Olive leaves, olives, virgin olive oil and olive mill waste [128] | DILI/ALD | ROS [129] |
| NASH | TLR [130] | |||
| Sesamin | Lignans | Flaxseed and sesame seeds [131] | Steatosis | ACC, CPT1, 3-hydroxyacyl-coA dehydrogenase [132] |
| Steatosis | SREBP-1 [133] | |||
| Diglucoside | Lignans | Flaxseed [134] | Steatosis/NASH | Lipoperoxidation [135] |
| Curcumin | Curcuminoids | Curcuma longa [136] | Steatosis | FAO [137] |
| Fibrosis/DILI/ALD | NRF-2, GSH, HSC activation [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón, J.; Casado-Andrés, M.; Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; Martínez-Chantar, M.L. Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients 2020, 12, 3517. https://doi.org/10.3390/nu12113517
Simón J, Casado-Andrés M, Goikoetxea-Usandizaga N, Serrano-Maciá M, Martínez-Chantar ML. Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients. 2020; 12(11):3517. https://doi.org/10.3390/nu12113517
Chicago/Turabian StyleSimón, Jorge, María Casado-Andrés, Naroa Goikoetxea-Usandizaga, Marina Serrano-Maciá, and María Luz Martínez-Chantar. 2020. "Nutraceutical Properties of Polyphenols against Liver Diseases" Nutrients 12, no. 11: 3517. https://doi.org/10.3390/nu12113517
APA StyleSimón, J., Casado-Andrés, M., Goikoetxea-Usandizaga, N., Serrano-Maciá, M., & Martínez-Chantar, M. L. (2020). Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients, 12(11), 3517. https://doi.org/10.3390/nu12113517