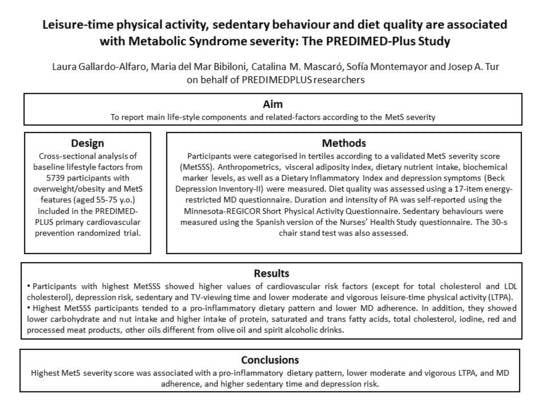
Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Participants, Recruitment, Randomization, and Ethics
2.3. Anthropometric and Blood Pressure Measurements
2.4. Blood Collection Analysis
2.5. Other Health Outcomes
2.6. Visceral Adiposity Index
2.7. Physical Activity and Sedentary Behaviour
2.8. Dietary Assessment
2.9. Assessment of Mediterranean Diet Adherence
2.10. Dietary Inflammatory Index
2.11. Metabolic Syndrome Severity Score
2.12. Statistics
3. Results
4. Discussion
4.1. Sociodemographic Factors
4.2. Cardiovascular Risk Factors
4.3. Depressive Symptoms
4.4. Sedentary Behaviour and Physical Activity
4.5. Dietary Characteristics
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| ANCOVA | analysis of covariance |
| ANOVA | analysis of variance; |
| BMI | body mass index |
| BP | blood pressure; |
| CHD | coronary heart disease |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| DII | dietary inflammatory index |
| FA | fatty acid |
| FFQ | food frequency questionnaire |
| HDL-c | high-density lipoprotein cholesterol |
| HR | heart rate |
| IQR | interquartile range |
| LDL-c | low-density lipoprotein cholesterol |
| MD | Mediterranean diet |
| MetS | Metabolic Syndrome |
| MetSSS | metabolic syndrome severity score |
| MUFA | monounsaturated fatty acids |
| NO | nitric oxide |
| PREDIMED | PREvención con DIeta MEDiterránea |
| PA | physical activity |
| PUFA | polyunsaturated fatty acids |
| RAPA | Rapid Assessment of Physical Activity Questionnaires |
| ROS | reactive oxygen species |
| SBP | systolic blood pressure |
| SD | standard deviations |
| SFA | saturated fatty acids |
| TAG | triglycerides |
| TFA | trans fatty acid |
| T2DM | type 2 diabetes mellitus |
| VAI | visceral adiposity index |
| WC | waist circumference |
| ω-3 FA | omega-3 fatty acids |
References
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 960, pp. 1–17. [Google Scholar]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the Metabolic Syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bergés, D.; Cabrera de León, A.; Sanz, H.; Elosua, R.; Guembe, M.J.; Alzamora, M.; Vega-Alonso, T.; Félix-Redondo, F.J.; Ortiz-Marrón, H.; Rigo, F.; et al. Metabolic syndrome in Spain: Prevalence and coronary risk associated with harmonized definition and WHO proposal. DARIOS study. Rev. Esp. Cardiol. 2012, 65, 241–248. [Google Scholar] [CrossRef]
- Bianchi, G.; Rossi, V.; Muscari, A.; Magalotti, D.; Zoli, M.; Berzigotti, A.; Pianoro Study Group. Physical activity is negatively associated with the metabolic syndrome in the elderly. QJM 2008, 101, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Galmes-Panades, A.M.; Varela-Mato, V.; Konieczna, J.; Wärnberg, J.; Martínez-González, M.Á.; Salas-Salvadó, J. Isotemporal substitution of inactive time with physical activity and time in bed: Cross-sectional associations with cardiometabolic health in the PREDIMED-Plus study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 137. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults a systematic review and meta-analysis. Annals of Internal Medicine. Am. Coll. Phys. 2015, 162, 123–132. [Google Scholar]
- Mankowski, R.T.; Aubertin-Leheudre, M.; Beavers, D.P.; Botoseneanu, A.; Buford, T.W.; Church, T.; Glynn, N.W.; King, A.C.; Liu, C.; Manini, T.M.; et al. Sedentary time is associated with the metabolic syndrome in older adults with mobility limitations—The LIFE Study. Exp. Gerontol. 2015, 70, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, A.; Keum, N.; Okereke, O.I.; Sun, Q.; Kivimaki, M.; Rubin, R.R.; Hu, F.B. Bidirectional Association Between Depression and Metabolic Syndrome. Diabetes Care 2012, 35, 1171–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors a Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martínez-González, M.A.; Castañer, O.; Bulló, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ 2014, 186, 649–657. [Google Scholar] [CrossRef] [Green Version]
- DeBoer, M.D.; Gurka, M.J.; Woo, J.G.; Morrison, J.A. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: The Princeton Lipid Research Cohort Study. Diabetologia 2015, 58, 2745–2752. [Google Scholar] [CrossRef] [Green Version]
- Wiley, J.F.; Carrington, M.J. A metabolic syndrome severity score: A tool to quantify cardio-metabolic risk factors. Prev. Med. (Baltim.) 2016, 88, 189–195. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.S., Jr. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of ObesityHarmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation 2009, 120, 640–645. [Google Scholar]
- Sanz, J.; Navarro, M.E.; Vázquez, C. Adaptación española para el Inventario de Depresión de Beck-II (BDI-II). 1. Propiedades psicométricas en estudiantes universitarios. Anál. Modif. Conducta 2003, 124, 239–288. [Google Scholar]
- Amato, M.C.; Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topolski, T.D.; LoGerfo, J.; Patrick, D.L.; Williams, B.; Walwick, J.; Patrick, M.B. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev. Chronic. Dis. 2006, 3, A118. [Google Scholar] [PubMed]
- Molina, L.; Sarmiento, M.; Peñafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schröder, H.; et al. Validation of the regicor short physical activity questionnaire for the adult population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.A.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- Moreiras, O.; Ángeles, C.; Luisa, C.; Cuadrado, C. Tabla de Composición de Alimentos; Pirámide: Madrid, Spain, 2007; pp. 37–46. [Google Scholar]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vázquez-Ruiz, Z.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferré, M.; Liu, X.; Malik, V.S.; Sun, Q.; Willett, W.C.; Manson, J.A.E.; Rexrode, K.M.; Li, Y.; Hu, F.B.; Bhupathiraju, S.N. Nut Consumption and Risk of Cardiovascular Disease. J. Am. Coll. Cardiol. 2017, 70, 2519–2532. [Google Scholar] [CrossRef]
- Whitman, I.R.; Agarwal, V.; Nah, G.; Dukes, J.W.; Vittinghoff, E.; Dewland, T.A.; Marcus, G.M. Alcohol Abuse and Cardiac Disease. J. Am. Coll. Cardiol. 2017, 69, 13–24. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Meat consumption and risk of metabolic syndrome: Results from the Korean population and a meta-analysis of observational studies. Nutrients 2018, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Hari, Y.; Nakashima, K.; Kuno, T.; Ando, T.; Alice (All-Literature Investigation of Cardiovascular Evidence) Group. Marriage and mortality after acute coronary syndrome. Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Santilli, F.; D’Ardes, D.; Guagnano, M.T.; Davi, G. Metabolic Syndrome: Sex-Related Cardiovascular Risk and Therapeutic Approach. Curr. Med. Chem. 2017, 24, 2602–2627. [Google Scholar] [CrossRef]
- Xue, B.; Head, J.; McMunn, A.; Heyn, P.C. The Impact of Retirement on Cardiovascular Disease and Its Risk Factors: A Systematic Review of Longitudinal Studies. Gerontologist 2019. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, T.C.; Chang, P.C.; Liu, C.S.; Lin, W.Y.; Wu, M.T.; Li, C.I.; Lin, C.C. Association among cigarette smoking, metabolic syndrome, and its individual components: The metabolic syndrome study in Taiwan. Metabolism 2008, 57, 544–548. [Google Scholar] [CrossRef]
- Syamlal, G.; Mazurek, J.M.; Dube, S.R. Gender differences in smoking among U.S. working adults. Am. J. Prev. Med. 2014, 47, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.; Song, Y.M.; Ko, H.; Sung, J.; Lee, K.; Shin, J.; Shin, S. Educational Disparities in Risk for Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, X.; Liu, Y.; Sun, X.; Han, C.; Zhang, L.; Wang, B.; Ren, Y.; Zhao, Y.; Zhang, D.; et al. Resting heart rate and risk of metabolic syndrome in adults: A dose–response meta-analysis of observational studies. Acta Diabetol. 2017, 54, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Womack, V.Y.; De Chavez, P.J.; Albrecht, S.S.; Durant, N.; Loucks, E.B.; Puterman, E.; Redmond, N.; Siddique, J.; Williams, D.R.; Carnethon, M.R. A Longitudinal Relationship between Depressive Symptoms and Development of Metabolic Syndrome: The Coronary Artery Risk Development in Young Adults Study. Psychosom. Med. 2016, 78, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Kazaz, I.; Angin, E.; Kabaran, S.; Iyigün, G.; Kirmizigil, B.; Malkoç, M. Evaluation of the physical activity level, nutrition quality, and depression in patients with metabolic syndrome. Medicine (Baltim.) 2018, 97, e0485. [Google Scholar] [CrossRef]
- Grøntved, A.; Hu, F.B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A meta-analysis. JAMA J. Am. Med. Assoc. 2011, 305, 2448–2455. [Google Scholar] [CrossRef] [Green Version]
- Bankoski, A.; Harris, T.B.; McClain, J.J.; Brychta, R.J.; Caserotti, P.; Chen, K.Y.; Berrigan, D.; Troiano, R.P.; Koster, A. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care 2011, 34, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Strasser, B. Physical activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 2013, 1281, 141–159. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Sánchez, J.A.; Fernández-Rodríguez, M.J.; Sanchis-Moysi, J.; del Rodríguez-Pérez, M.C.; Marcelino-Rodríguez, I.; de León, A.C. Domain and intensity of physical activity are associated with metabolic syndrome: A population-based study. PLoS ONE 2019, 14, e0219798. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.M.; Lancet Physical Activity Series 2 Executive Committe; Lancet Sedentary Behaviour Working Group. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Pedrero-Chamizo, R.; Gómez-Cabello, A.; Delgado, S.; Rodríguez-Llarena, S.; Rodríguez-Marroyo, J.A.; Cabanillas, E.; Meléndez, A.; Vicente-Rodríguez, G.; Aznar, S.; Villa, G.; et al. Physical fitness levels among independent non-institutionalized Spanish elderly: The elderly EXERNET multi-center study. Arch. Gerontol. Geriatr. 2012, 55, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namazi, N.; Larijani, B.; Azadbakht, L. Dietary Inflammatory Index and its Association with the Risk of Cardiovascular Diseases, Metabolic Syndrome, and Mortality: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2018, 50, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Abete, I.; Romaguera, D.; Vieira, A.R.; De Lopez Munain, A.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef]
- Mirmiran, P.; Ziadlou, M.; Karimi, S.; Hosseini-Esfahani, F.; Azizi, F. The association of dietary patterns and adherence to WHO healthy diet with metabolic syndrome in children and adolescents: Tehran lipid and glucose study. BMC Public Health 2019, 19, 1457. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2015, CD011737. [Google Scholar] [CrossRef]
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2019. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, 128308. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpour-Niazi, S.; Hosseini, S.; Mirmiran, P.; Azizi, F. Prospective study of nut consumption and incidence of metabolic syndrome: Tehran Lipid and glucose study. Nutrients 2017, 9, 1056. [Google Scholar] [CrossRef] [Green Version]
- Soriguer, F.; García-Fuentes, E.; Gutierrez-Repiso, C.; Rojo-Martínez, G.; Velasco, I.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle, A.; Carmena, R.; et al. Iodine intake in the adult population. [email protected] study. Clin. Nutr. 2012, 31, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Sparks, E.; Farrand, C.; Santos, J.A.; McKenzie, B.; Trieu, K.; Reimers, J.; Davidson, C.; Johnson, C.; Webster, J. Sodium Levels of Processed Meat in Australia: Supermarket Survey Data from 2010 to 2017. Nutrients 2018, 10, 1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, J.; Guasch-Ferré, M.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Fitó, M.; Ros, E.; Arós, F.; Bulló, M.; Gómez-Gracia, E.; et al. Is complying with the recommendations of sodium intake beneficial for health in individuals at high cardiovascular risk? Findings from the PREDIMED study. Am. J. Clin. Nutr. 2015, 101, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, G.; Mozzillo, E.; Zito, E.; De Nitto, E.; Maltoni, G.; Marigliano, M.; Zucchini, S.; Maffeis, C.; Franzese, A. Alcohol consumption or cigarette smoking and cardiovascular disease risk in youth with type 1 diabetes. Acta Diabetol. 2019, 56, 1315–1321. [Google Scholar] [CrossRef]

| Variables | Tertile 1 (n = 1913) | Tertile 2 (n = 1913) | Tertile 3 (n = 1913) | p-Value |
|---|---|---|---|---|
| Women, n (%) | 704 (36.8) | 981 (51.3) | 1054 (55.1) | <0.001 |
| Age, years | 64.7 ± 5.1 a | 65.3 ± 4.9 a | 65.0 ± 4.9 | <0.001 |
| Prevalence of obesity, n (%) | 1000 (52.3) | 1535 (80.2) | 1647 (86.1) | <0.001 |
| Education level, n (%) | 1899 | 1893 | 1895 | <0.001 |
| Illiterate or primary education | 846 (44.5) | 945 (49.9) | 992 (52.3) | |
| Secondary education | 573 (30.2) | 552 (29.2) | 524 (27.7) | |
| Academic or graduate | 480 (25.3) | 396 (20.9) | 379 (20.0) | |
| Smoking habit, n (%) | 1904 | 1905 | 1909 | <0.001 |
| Never smoked | 749 (39.3) | 852 (44.7) | 904 (47.4) | |
| Former smoker | 907 (47.6) | 791 (41.5) | 795 (41.6) | |
| Current smoker | 248 (13.0) | 262 (13.8) | 210 (11.0) | |
| Married status | 1902 | 1906 | 1910 | 0.012 |
| Single or divorced | 242 (12.7) | 261 (13.7) | 261 (13.7) | |
| Married | 1499 (78.8) | 1442 (75.7) | 1427 (74.7) | |
| Widower | 161 (8.5) | 203 (10.7) | 222 (11.6) | |
| Employment status, n (%) | 1902 | 1904 | 1895 | <0.001 |
| Working | 455 (23.9) | 342 (18.0) | 367 (19.4) | |
| Non-working | 372 (19.6) | 435 (22.8) | 508 (26.8) | |
| Retired | 1075 (56.5) | 1127 (59.2) | 1020 (53.8) | |
| MetS components, n (%) | ||||
| High blood pressure Hyperglycaemia Hypertriglyceridemia Low HDL-cholesterol Abdominal obesity | 1719 (89.9) 1322 (69.1) 1065 (55.7) 742 (38.8) 1769 (92.5) | 1750 (91.5) 1435 (75.0) 1072 (56.0) 837 (43.8) 1859 (97.2) | 1801 (94.1) 1558 (81.4) 1102 (57.6) 903 (47.2) 1876 (98.1) | <0.001 <0.001 0.440 <0.001 <0.001 |
| Medication | ||||
| Antihypertensive agents | 1460 (76.3) | 1466 (76.6) | 1539 (80.4) | 0.003 |
| Anti-cholesterol agents | 966 (50.5) | 964 (50.4) | 1053 (55.0) | 0.018 |
| Insulin | 36 (1.9) | 64 (3.3) | 174 (9.1) | <0.001 |
| Oral hypoglycaemic agents | 271 (14.2) | 433 (22.6) | 802 (41.9) | <0.001 |
| Aspirin or antiplatelet drugs | 240 (12.5) | 278 (14.5) | 376 (19.7) | <0.001 |
| Variables | Tertile 1 (n = 1913) | Tertile 2 (n = 1913) | Tertile 3 (n = 1913) | p-Value | Sex and Age Adjusted p-Value | Full Adjusted p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||||
| Weight, kg | 81.6 ± 11.0 a,b | 81.2 (16.1) | 86.5 ± 11.9 a,c | 85.3 (16.9) | 91.6 ± 13.9 b,c | 90.6 (19.0) | <0.001 | <0.001 | <0.001 |
| BMI, kg/m2 | 30.4 ± 2.3 a,b | 30.1 (3.3) | 32.7 ± 3.0 a,c | 32.5 (4.3) | 34.4 ± 3.6 b,c | 34.6 (5.7) | <0.001 | <0.001 | <0.001 |
| Waist circumference, cm | 101.7 ± 7.2 a,b | 102.0 (10.7) | 107.7 ± 7.7 a,c | 107.0 (10.6) | 113.1 ± 10.0 b,c | 113.0 (13.3) | <0.001 | <0.001 | <0.001 |
| Women | 94.8 ± 4.3 a,b | 95.0 (6.5) | 103.4 ± 5.0 a,c | 104.0 (6.4) | 110.4 ± 9.0 b,c | 112.0 (11.4) | <0.001 | <0.001 | <0.001 |
| Men | 105.7 ± 5.3 a,b | 106.0 (7.4) | 112.3 ± 7.4 a,c | 113.5 (11.0) | 116.4 ± 10.1 b,c | 116.0 (16.1) | <0.001 | <0.001 | <0.001 |
| Glucose, mg/dL | 101.6 ± 12.5 a,b | 101.0 (17.0) | 109.3 ± 17.8 a,c | 106.0 (24.0) | 129.4 ± 40.4 b,c | 118.0 (48.0) | <0.001 | <0.001 | <0.001 |
| Glycated haemoglobin,% | 5.8 ± 0.5 a,b | 5.7 (0.5) | 6.0 ± 0.6 a,c | 5.9 (0.7) | 6.5 ± 1.2 b,c | 6.2 (1.4) | <0.001 | <0.001 | <0.001 |
| Total cholesterol, mg/dL | 196.0 ± 35.8 | 195.0 (50.0) | 197.8 ± 38.2 | 196.0 (52.0) | 194.3 ± 38.4 | 192.0 (51.0) | <0.001 | <0.001 | <0.001 |
| HDL-cholesterol, mg/dL | 49.2 ± 12.0 a,b | 47.0 (15.0) | 47.8 ± 11.4 a | 46.0 (14.0) | 46.8 ± 12.1 b | 46.0 (15.0) | <0.001 | <0.001 | <0.001 |
| LDL-cholesterol, mg/dL | 121.8 ± 41.2 | 119.0 (43.0) | 122.8 ± 46.3 | 120.0 (44.0) | 117.4 ± 40.5 | 114.0 (44.0) | <0.001 | <0.001 | <0.001 |
| Triglycerides, mg/dL | 133.4 ± 49.5 a,b | 127.0 (68.0) | 151.0 ± 64.1 a,c | 138.0 (77.0) | 171.8 ± 103.3 b,c | 144.0 (94.0) | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure, mmHg | 134.1 ± 14.2 a,b | 133.3 (18.3) | 138.5 ± 16.3 a,c | 137.3 (20.7) | 143.9 ± 18.8 b,c | 142.0 (23.7) | <0.001 | <0.001 | <0.001 |
| Diastolic blood pressure, mmHg | 80.1 ± 8.4 a,b | 80.3 (11.0) | 81.7 ± 9.5 a,c | 81.7 (12.7) | 84.1 ± 10.8 b,c | 83.3 (15.0) | <0.001 | <0.001 | <0.001 |
| Heart rate, bpm | 68.0 ± 9.9 a,b | 67.3 (13.0) | 70.3 ± 10.5 a,c | 69.7 (14.0) | 73.0 ± 11.2 b,c | 72.0 (14.9) | <0.001 | <0.001 | <0.001 |
| Visceral adiposity index | 2.0 ± 0.9 a,b | 1.8 (1.3) | 2.5 ± 1.4 a,c | 2.2 (1.6) | 3.0 ± 2.5 b,c | 2.4 (2.1) | <0.001 | <0.001 | <0.001 |
| Beck Depression Inventory-II | 7.5 ± 6.8 b | 6.0 (9.0) | 8.4 ± 7.4 c | 7.0 (9.0) | 9.5 ± 7.9 b,c | 8.0 (9.0) | <0.001 | <0.001 | <0.001 |
| Varibles | Tertile 1 (n = 1913) | Tertile 2 (n = 1913) | Tertile 3 (n = 1913) | p-Value | Sex and Age Adjusted p-Value | Full Adjusted p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||||
| Sedentary time, h/d | 7.9 ± 1.9 a,b | 8.0 (2.7) | 8.0 ± 1.9 a | 8.0 (2.9) | 8.2 ± 2.0 b | 8.0 (3.3) | <0.001 | <0.001 | <0.001 |
| TV-viewing time, h/d | 5.0 ± 1.7 b | 5.0 (2.0) | 5.2 ± 1.8 | 5.0 (2.3) | 5.3 ± 1.9 b | 5.0 (2.4) | <0.001 | <0.001 | 0.003 |
| Sleeping time, h/d | 7.0 ± 1.2 | 7.0 (2.0) | 7.1 ± 1.2 | 7.0 (2.0) | 7.0 ± 1.3 | 7.0 (2.0) | 0.128 | 0.056 | 0.081 |
| Total LTPA, MET·min/d | 378.4 ± 282.3 a,b | 311.7 (360.8) | 335.0 ± 272.4 a | 267.2 (353.3) | 307.3 ± 261.1 b | 239.8 (303.7) | <0.001 | <0.001 | <0.001 |
| Light LTPA, MET·min/d | 115.3 ± 140.9 | 71.9 (159.8) | 110.3 ± 132.6 | 63.9 (159.8) | 110.7 ± 126.3 | 79.9 (151.9) | 0.433 | 0.588 | 0.417 |
| Moderate LTPA, MET·min/d | 147.7 ± 195.7 b | 74.9 (224.8) | 128.0 ± 183.9 | 40.0 (199.8) | 111.0 ± 181.9 b | 10.0 (159.8) | <0.001 | <0.001 | <0.001 |
| Vigorous LTPA, MET·min/d | 115.4 ± 184.8 a,b | 18.0 (163.3) | 96.7 ± 164.8 a | 12.0 (137.2) | 85.7 ± 155.8 b | 8.0 (105.6) | <0.001 | <0.001 | <0.001 |
| 30-s chair stand test, n | 14.1 ± 5.0 a,b | 14.0 (6.0) | 13.2 ± 5.0 a,c | 13.0 (5.0) | 12.5 ± 4.9 b,c | 12.0 (5.0) | <0.001 | <0.001 | <0.001 |
| Vriables | Tertile 1 (n = 1913) | Tertile 2 (n = 1913) | Tertile 3 (n = 1913) | p-Value | Sex and Age Adjusted p-Value | Full Adjusted p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||||
| Total energy (TE), kcal/d | 2392.7 ± 547.8 | 2354.1 (724.4) | 2359.0 ± 553.9 | 2329.5(758.7) | 2345.7 ± 549.1 | 2321.2 (764.6) | 0.024 | 0.733 | 0.640 |
| Total fat intake, %TE | 39.0 ± 6.3 a,b | 39.0 (8.7) | 39.8 ± 6.6 a | 39.8 (8.7) | 39.6 ± 6.5 b | 39.6 (9.1) | 0.001 | 0.002 | 0.003 |
| MUFA, % TE | 20.2 ± 4.6 a | 20.1 (6.0) | 20.7 ± 4.7 a | 20.6 (6.4) | 20.4 ± 4.6 | 20.2 (6.5) | 0.005 | 0.012 | 0.023 |
| PUFA, %TE | 6.4 ± 1.8 | 6.1 (2.2) | 6.4 ± 1.8 | 6.1 (2.3) | 6.3 ± 1.9 | 6.0 (2.2) | 0.450 | 0.415 | 0.457 |
| SFA,%TE | 9.8 ± 2.0 a,b | 9.6 (2.6) | 10.0 ± 2.0 a | 9.9 (2.5) | 10.1 ± 2.0 b | 10.0 (2.6) | 0.001 | <0.001 | <0.001 |
| Trans FA, g/d | 0.6 ± 0.4 b | 0.5 (0.5) | 0.6 ± 0.4 | 0.5 (0.5) | 0.6 ± 0.4 b | 0.5 (0.5) | 0.225 | 0.056 | 0.029 |
| Linoleic acid, g/d | 13.8 ± 5.6 | 12.8 (7.0) | 13.6 ± 5.5 | 12.7 (7.3) | 13.4 ± 5.7 | 12.3 (6.8) | 0.194 | 0.805 | 0.830 |
| w-3 FA, g/d | 2.4 ± 0.9 | 2.3 (1.3) | 2.4 ± 1.0 | 2.2 (1.3) | 2.3 ± 0.9 | 2.1 (1.2) | 0.005 | 0.014 | 0.049 |
| Carbohydrate intake, %TE | 41.1 ± 6.7 a,b | 41.1 (9.4) | 40.4 ± 6.9 a | 40.4 (9.2) | 40.5 ± 6.9 b | 40.5 (9.4) | 0.008 | <0.001 | <0.001 |
| Glycaemic index | 54.1 ± 5.1 | 54.4 (6.8) | 53.7 ± 5.2 | 54.1 (6.8) | 53.9 ± 5.1 | 54.2 (6.7) | 0.146 | 0.367 | 0.557 |
| Protein intake, %TE | 16.5 ± 2.8 b | 16.2 (3.6) | 16.8 ± 2.8 | 16.7 (3.7) | 17.1 ± 2.9 b | 16.9 (3.6) | <0.001 | 0.002 | 0.003 |
| Cholesterol, mg/d | 382.1 ± 118.8 b | 369.5 (136.9) | 380.3 ± 119.3 | 168.8 (143.3) | 386.4 ± 113.4 b | 374.5 (135.9) | 0.251 | 0.013 | 0.006 |
| Fibre intake, g/d | 26.3 ± 9.0 | 24.7 (11.0) | 26.3 ± 9.0 | 25.0 (11.0) | 25.9 ± 8.4 | 25.0 (10.6) | 0.300 | 0.061 | 0.094 |
| Alcohol intake, g/d | 12.3 ± 15.3 | 6.2 (15.4) | 10.4 ± 14.1 | 4.7 (13.0) | 10.4 ± 15.6 | 4.3 (11.9) | <0.001 | 0.509 | 0.457 |
| Vitamin A, µg/d | 1111.9 ± 639.7 | 936.8 (781.5) | 1098.8 ± 654.4 | 929.0 (753.1) | 1120.0 ± 638.8 | 936.7 (766.4) | 0.592 | 0.583 | 0.480 |
| Vitamin B1, mg/d | 1.6 ± 0.4 | 1.6 (0.5) | 1.6 ± 0.4 | 1.6 (0.6) | 1.6 ± 0.4 | 1.6 (0.5) | 0.935 | 0.840 | 0.742 |
| Vitamin B2, mg/d | 2.0 ± 0.6 | 1.9 (0.8) | 2.0 ± 0.7 | 1.9 (0.8) | 2.0 ± 0.6 | 1.9 (0.8) | 0.271 | 0.619 | 0.497 |
| Vitamin B3, mg/d | 40.6 ± 10.0 | 40.0 (13.8) | 40.8 ± 10.0 | 40.4 (12.9) | 41.0 ± 9.9 | 40.5(12.7) | 0.386 | 0.219 | 0.151 |
| Vitamin B6, mg/d | 2.4 ± 0.6 | 2.3 (0.8) | 2.4 ± 0.6 | 2.3 (0.8) | 2.3 ± 0.6 | 2.3 (0.8) | 0.283 | 0.237 | 0.344 |
| Vitamin B9, µg/d | 352.5 ± 104.8 | 336.8 (134.3) | 353.5 ± 103.7 | 338.1 (126.7) | 349.3 ± 99.4 | 339.0 (125.8) | 0.419 | 0.222 | 0.279 |
| Vitamin B12, µg/d | 10.0 ± 4.6 | 9.2 (5.8) | 10.0 ± 4.4 | 9.1(5.5) | 10.1 ± 4.5 | 9.2(5.6) | 0.400 | 0.200 | 0.212 |
| Vitamin C, mg/d | 200.2 ± 87.8 | 184.5(108.1) | 204.7 ± 86.3 | 188.6(114.7) | 200.2 ± 83.7 | 187.0 (104.6) | 0.166 | 0.138 | 0.156 |
| Vitamin D, µg/d | 6.3 ± 3.5 | 5.2 (5.4) | 6.2 ± 3.4 | 5.2 (5.4) | 6.1 ± 3.5 | 5.0 (5.0) | 0.230 | 0.189 | 0.232 |
| Vitamin E, mg/d | 10.6 ± 4.0 | 10.0 (4.3) | 10.6 ± 3.9 | 10.0 (4.3) | 10.6 ± 4.1 | 9.9 (4.3) | 0.966 | 0.733 | 0.646 |
| Calcium, mg/d | 1026.7 ± 339.7 | 984.8 (431.9) | 1040.9 ± 348.3 | 997.3 (453.8) | 1038.1 ± 350.2 | 997.4 (449.6) | 0.409 | 0.475 | 0.320 |
| Phosphorus, mg/d | 1749.5 ± 411.4 | 1715.6 (540.9) | 1765.1 ± 431.6 | 1733.7 (597.4) | 1769.1 ± 422.0 | 1736.1 (558.2) | 0.315 | 0.294 | 0.149 |
| Magnesium, mg/d | 423.1 ± 109.0 | 409.5 (142.6) | 422.6 ± 111.2 | 410.9 (143.9) | 417.1 ± 106.3 | 405.5 (145.7) | 0.171 | 0.241 | 0.372 |
| Iron, mg/d | 16.6 ± 4.0 | 16.3 (5.4) | 16.5 ± 4.0 | 16.2 (5.1) | 16.4 ± 3.9 | 16.1 (5.2) | 0.355 | 0.958 | 0.999 |
| Iodine, µg/d | 274.1 ± 154.8 b | 256.5 (130.5) | 288.3 ± 162.7 | 258.9 (140.4) | 290.8 ± 158.8 b | 259.7 (125.6) | 0.002 | 0.030 | 0.019 |
| Potassium, mg/d | 4457.1 ± 1067.4 | 4346.5 (1401.0) | 4493.8 ± 1101.2 | 4396.4 (1441.6) | 4466.5 ± 1077.9 | 4365.2 (1374.5) | 0.552 | 0.686 | 0.700 |
| Selenium, µg/d | 117.6 ± 33.4 | 114.9 (42.7) | 116.6 ± 33.4 | 114.2 (44.5) | 117.6 ± 32.4 | 115.4 (43.1) | 0.545 | 0.428 | 0.280 |
| Zinc, mg/d | 13.2 ± 3.3 | 12.9 (4.3) | 13.2 ± 3.3 | 12.9 (4.5) | 13.2 ± 3.2 | 13.0 (4.2) | 0.969 | 0.400 | 0.257 |
| Sodium, mg/d | 2427.8 ± 760.3 b | 2332.5 (957.0) | 2406.6 ± 794.1 c | 2312.1 (1008.0) | 2459.4 ± 775.8 b,c | 2371.3 (976.7) | 0.106 | 0.001 | <0.001 |
| Variables | Tertile 1 (n = 1913) | Tertile 2 (n = 1913) | Tertile 3 (n = 1913) | p-Value | Sex and Age Adjusted p-Value | Full Adjusted p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||||
| Food groups | |||||||||
| Fruits, g/d | 352.3 ± 203.4 | 320.4 (245.1) | 361.6 ± 204.9 | 331.8 (246.2) | 358.0 ± 210.0 | 325.6 (252.0) | 0.373 | 0.880 | 0.863 |
| Vegetables, g/d | 324.5 ± 138.1 | 304.3 (181.9) | 329.6 ± 143.2 | 306.5 (170.8) | 328.7 ± 139.7 | 306.9 (177.8) | 0.487 | 0.825 | 0.758 |
| Potatoes and tubers, g/d | 64.9 ± 42.5 | 50.0 (67.1) | 65.7 ± 43.0 | 50.0 (67.1) | 65.2 ± 43.5 | 50.0 (67.1) | 0.858 | 0.789 | 0.746 |
| Legumes, g/d | 21.0 ± 11.0 | 20.0 (9.7) | 20.8 ± 11.7 | 20.6 (13.1) | 20.6 ± 11.1 | 17.1 (12.6) | 0.670 | 0.205 | 0.811 |
| Nuts, g/d | 15.9 ± 17.9 b | 10.3 (21.7) | 15.3 ± 17.6 c | 8.6 (23.6) | 13.5 ± 16.5 b,c | 8.0 (17.1) | <0.001 | <0.001 | <0.001 |
| Cereals, g/d | 153.4 ± 79.3 | 126.4 (112.5) | 147.8 ± 77.3 | 119.7 (116.0) | 150.3 ± 74.5 | 126.4 (112.5) | 0.080 | 0.411 | 0.441 |
| Whole cereals | 41.9 ± 64.1 | 5.0 (75.0) | 42.3 ± 63.6 | 5.0 (75.0) | 41.1 ± 62.4 | 4.0 (75.0) | 0.831 | 0.173 | 0.307 |
| Refined cereals | 111.5 ± 89.1 | 92.1 (162.1) | 105.5 ± 85.5 | 87.6 (161.2) | 109.3 ± 85.5 | 92.1 (160.7) | 0.097 | 0.110 | 0.185 |
| Fish and seafood, g/d | 101.9 ± 47.0 | 97.0 (64.0) | 103.5 ± 49.3 | 99.5 (65.3) | 102.0 ± 47.5 | 96.0 (62.1) | 0.504 | 0.557 | 0.757 |
| White fish | 38.9 ± 25.6 | 25.4 (42.9) | 40.3 ± 27.0 | 25.4 (42.9) | 40.4 ± 26.4 | 25.4 (42.9) | 0.160 | 0.690 | 0.570 |
| Bluefish | 35.0 ± 22.8 | 25.7 (37.1) | 34.7 ± 22.5 | 25.7 (38.2) | 34.0 ± 22.7 | 25.7 (34.1) | 0.354 | 0.253 | 0.311 |
| Seafood | 28.0 ± 21.2 | 30.6 (17.3) | 28.6 ± 23.8 | 30.6 (17.3) | 27.6 ± 21.3 | 30.6 (17.3) | 0.425 | 0.321 | 0.376 |
| Total meat, g/d | 144.7 ± 59.8 b,a | 138.5 (71.1) | 147.2 ± 57.4 c,a | 143.0 (70.8) | 152.0 ± 59.0 b,c | 146.9 (74.7) | <0.001 | <0.001 | <0.001 |
| Red meat | 48.5 ± 35.1 a,b | 41.4 (46.2) | 49.5 ± 34.0a | 41.4 (46.2) | 49.4 ± 34.2 b | 42.8 (50.0) | 0.596 | 0.004 | 0.002 |
| White meat | 60.3 ± 33.5 | 64.3 (42.9) | 61.6 ± 33.8 | 64.3 (42.9) | 64.2 ± 35.7 | 64.3 (52.9) | 0.002 | 0.029 | 0.066 |
| Processed meat | 33.8 ± 22.0 b | 30.6 (23.0) | 34.1 ± 22.5 c | 31.3 (24.2) | 36.2 ± 24.9 b,c | 32.4 (25.1) | 0.002 | <0.001 | <0.001 |
| Viscera | 2.2 ± 5.3 | 0.0 (0.0) | 2.1 ± 5.4 | 0.0 (0.0) | 2.3 ± 5.3 | 0.0 (0.0) | 0.517 | 0.295 | 0.328 |
| Eggs | 24.0 ± 12.4 | 25.7 (0.0) | 23.6 ± 12.0 | 25.7 (0.0) | 24.2 ± 11.5 | 25.7 (0.0) | 0.300 | 0.190 | 0.155 |
| Dairy products, g/d | 332.5 ± 194.4 | 298.2 (189.4) | 349.3 ± 203.3 | 310.2 (265.0) | 351.6 ± 206.4 | 304.6 (297.9) | 0.006 | 0.125 | 0.077 |
| Whole-fat dairy | 45.5 ± 97.1 | 0.0 (53.6) | 43.8 ± 96.5 | 0.0 (53.6) | 45.3 ± 97.9 | 0.0 (53.6) | 0.829 | 0.522 | 0.541 |
| Skimmed dairy | 146.9 ± 160.5 | 125.0 (200.0) | 156.8 ± 170.8 | 125.0 (209.5) | 155.4 ± 175.1 | 111.5 (209.5) | 0.147 | 0.572 | 0.471 |
| Cheese | 29.8 ± 25.1 | 24.8 (32.4) | 30.5 ± 24.6 | 24.8 (28.8) | 30.9 ± 25.0 | 25.0 (29.1) | 0.387 | 0.470 | 0.287 |
| Dairy desserts | 16.0 ± 30.2 | 6.7 (15.3) | 14.4 ± 25.5 | 6.7 (15.3) | 15.2 ± 32.5 | 6.7 (15.3) | 0.272 | 0.574 | 0.534 |
| Cookies and sweets, g/d | 26.9 ± 29.5 | 18.9 (32.7) | 25.6 ± 28.6 c | 15.5 (30.7) | 27.5 ± 30.7 c | 17.8 (33.7) | 0.108 | 0.081 | 0.041 |
| Olive oil, g/day | 39.2 ± 17.1 | 50.0 (25.0) | 40.2 ± 16.8 | 50.0 (25.0) | 39.0 ± 17.1 | 50.0 (25.0) | 0.048 | 0.030 | 0.054 |
| Other oils and fats, g/d | 2.8 ± 6.2 b | 0.7 (2.2) | 2.8 ± 6.2 c | 0.7 (3.0) | 3.4 ± 7.0 b,c | 0.8 (4.3) | 0.004 | 0.003 | 0.007 |
| Ready-to-eat-meals/Snacks, g/d | 10.9 ± 14.1 | 5.3 (16.0) | 10.7 ± 14.8 | 4.3 (16.7) | 11.0 ± 14.6 | 5.3 (16.0) | 0.790 | 0.306 | 0.195 |
| Alcoholic drinks, ml/d | |||||||||
| Wine | 67.9 ± 107.5 | 14.3 (100.0) | 54.3 ± 96.1 | 14.3 (78.6) | 53.7 ± 100.7 | 6.7 (49.5) | <0.001 | 0.413 | 0.437 |
| Beer | 119.9 ± 216.8 | 22.0 (141.4) | 112.0 ± 224.5 | 22.0 (141.4) | 102.8 ± 222.4 | 22.0 (141.4) | 0.056 | 0.226 | 0.294 |
| Spirits | 3.7 ± 10.8 b | 0.0 (3.3) | 3.0 ± 9.5 c | 0.0 (3.3) | 3.9 ± 12.6 b,c | 0.0 (3.3) | 0.020 | 0.001 | 0.001 |
| Dietary Inflammatory Index | −0.1 ± 2.0 | −0.1 (3.1) | 0.0 ± 2.1 | 0.0 (3.0) | 0.1 ± 2.0 | 0.1 (3.1) | 0.103 | 0.035 | 0.057 |
| Mediterranean Diet Adherence | 8.5 ± 2.7 | 8.0 (4.0) | 8.5 ± 2.7 | 8.0 (3.0) | 8.4 ± 2.5 | 8.0 (3.0) | 0.345 | 0.037 | 0.068 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Alfaro, L.; Bibiloni, M.d.M.; Mascaró, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients 2020, 12, 1013. https://doi.org/10.3390/nu12041013
Gallardo-Alfaro L, Bibiloni MdM, Mascaró CM, Montemayor S, Ruiz-Canela M, Salas-Salvadó J, Corella D, Fitó M, Romaguera D, Vioque J, et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients. 2020; 12(4):1013. https://doi.org/10.3390/nu12041013
Chicago/Turabian StyleGallardo-Alfaro, Laura, Maria del Mar Bibiloni, Catalina M. Mascaró, Sofía Montemayor, Miguel Ruiz-Canela, Jordi Salas-Salvadó, Dolores Corella, Montserrat Fitó, Dora Romaguera, Jesús Vioque, and et al. 2020. "Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study" Nutrients 12, no. 4: 1013. https://doi.org/10.3390/nu12041013
APA StyleGallardo-Alfaro, L., Bibiloni, M. d. M., Mascaró, C. M., Montemayor, S., Ruiz-Canela, M., Salas-Salvadó, J., Corella, D., Fitó, M., Romaguera, D., Vioque, J., Alonso-Gómez, Á. M., Wärnberg, J., Martínez, J. A., Serra-Majem, L., Estruch, R., Fernández-García, J. C., Lapetra, J., Pintó, X., García Ríos, A., ... Tur, J. A., on behalf of the PREDIMED-Plus Investigators. (2020). Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients, 12(4), 1013. https://doi.org/10.3390/nu12041013