Wheat Consumption Leads to Immune Activation and Symptom Worsening in Patients with Familial Mediterranean Fever: A Pilot Randomized Trial
Abstract
:1. Introduction
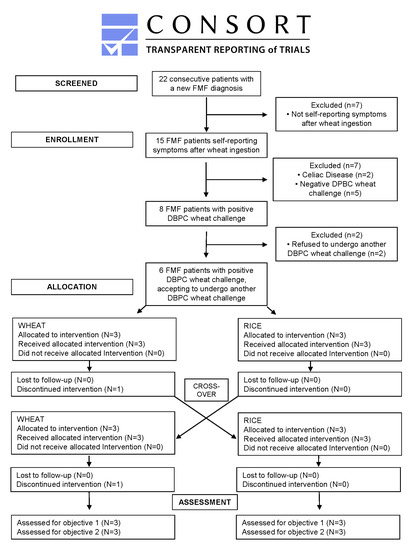
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Serum Markers of Inflammation
3.3. Immune Profiling of PBMC by FACS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Registration
Abbreviations
| AIDAI | Auto-Inflammatory Disease Activity Index |
| CeD | Celiac Disease |
| CRP | C-Reactive Protein |
| DBPC | Double-Blind Placebo-Controlled |
| FABP2 | Fatty Acid-Binding Protein 2 |
| FMF | Familial Mediterranean Fever |
| LBP | Lipopolysaccharide-Binding Protein |
| NCWS | Non-Celiac Wheat Sensitivity |
| PBMC | Peripheral Blood Monocytes Cells |
| SAA | Serum Amyloid A |
| sCD14 | soluble CD14 |
| SD | Standard Deviation |
References
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC. Med. 2012. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; De Magistris, L.; Dolinsek, J. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, A.; Akyüz, F.; Göktürk, S.; Evirgen, S.; Akyüz, U.; Örmeci, A.; Soyer, O.; Karaca, C.; Demir, K.; Gundogdu, G. Small bowel mucosal damage in familial Mediterranean fever: Results of capsule endoscopy screening. Scand. J. Gastroenterol. 2014, 49, 1414–1418. [Google Scholar] [CrossRef]
- Berkun, Y.; Eisenstein, E.M. Diagnostic criteria of familial Mediterranean fever. Autoimmun. Rev. 2014, 13, 388–390. [Google Scholar] [CrossRef]
- Zemer, D.; Pras, M.; Sohar, E.; Gafni, J. Letter: Colchicine in familial Mediterranean fever. N. Engl. J. Med. 1976, 294, 170–171. [Google Scholar] [CrossRef]
- Livneh, A.; Langevitz, P.; Zemer, D.; Zaks, N.; Kees, S.; Lidar, T.; Migdal, A.; Padeh, S.; Pras, M. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997, 40, 1879–1885. [Google Scholar] [CrossRef]
- Carroccio, A.; Giannone, G.; Mansueto, P.; Soresi, M.; La Blasca, F.; Fayer, F.; Iacobucci, R.; Porcasi, R.; Catalano, T.; Geraci, G. Duodenal and Rectal Mucosa Inflammation in Patients With Non-celiac Wheat Sensitivity. Clin. Gastroenterol. Hepatol. 2019, 17, 682–690. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.K.; Maxeiner, J.; Scholtes, P.; Steinbrink, K.; Schuppan, D. Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur. J. Nutr. 2019, 58, 1507–1514. [Google Scholar] [CrossRef]
- Piram, M.; Koné-Paut, I.; Lachmann, H.J.; Frenkel, J.; Ozen, S.; Kuemmerle-Deschner, J.; Stojanov, S.; Simon, A.; Finetti, M.; Sormani, M.P. Validation of the auto-inflammatory diseases activity index (AIDAI) for hereditary recurrent fever syndromes. Ann. Rheum. Dis. 2014, 73, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Linaker, B.D. Non Celiac Gluten Sensitivity. Lancet. 1978, 1, 1386–1389. [Google Scholar] [CrossRef]
- Mansueto, P.; Seidita, A.; D’Alcamo, A.; Carroccio, A. Non-celiac gluten sensitivity: Literature review. J. Am. Coll. Nutr. 2014, 33, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Villalta, D.; Roncoroni, L.; Barisani, D.; Ferrero, S.; Pellegrini, N.; Bardella, M.T.; Valiante, F.; Tomba, C.; Carroccio, A. Nomenclature and diagnosis of gluten-related disorders: A position statement by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO). Dig. Liver Dis. 2017, 49, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volta, U.; Caio, G.; Karunaratne, T.B.; Alaedini, A.; De Giorgio, R. Non-coeliac gluten/wheat sensitivity: Advances in knowledge and relevant questions. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 9–18. [Google Scholar] [CrossRef]
- Carroccio, A.; D’Alcamo, A.; Cavataio, F.; Soresi, M.; Seidita, A.; Sciumè, C.; Geraci, G.; Iacono, G.; Mansueto, P. High Proportions of People With Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Gastroenterology 2015, 149, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Portincasa, P.; Scaccianoce, G.; Palasciano, G. Familial mediterranean fever: A fascinating model of inherited autoinflammatory disorder. Eur. J. Clin. Investig. 2013, 43, 1314–1327. [Google Scholar] [CrossRef]
- Stojanov, S.; Kastner, D.L. Familial autoinflammatory diseases: Genetics, pathogenesis and treatment. Curr. Opin. Rheumatol. 2005, 17, 586–599. [Google Scholar] [CrossRef]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC. Med. 2011, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Ozen, S.; Batu, E.D. The myths we believed in familial Mediterranean fever: What have we learned in the past years? Semin. Immunopathol. 2015, 37, 363–369. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [Green Version]
- Caminero, A.; McCarville, J.L.; Zevallos, V.F.; Pigrau, M.; Yu, X.B.; Jury, J.; Galipeau, H.J.; Clarizio, A.V.; Casqueiro, J.; Murray, J.A. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology 2019, 156, 2266–2280. [Google Scholar] [CrossRef] [Green Version]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Bellinghausen, I.; Weigmann, B.; Zevallos, V.; Maxeiner, J.; Reißig, S.; Waisman, A.; Schuppan, D.; Saloga, J. Wheat amylase-trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice. J. Allergy Clin. Immunol. 2019, 143, 201–212. [Google Scholar] [CrossRef]
- Schuppan, D.; Pickert, G.; Ashfaq-Khan, M.; Zevallos, V. Non-celiac wheat sensitivity: Differential diagnosis, triggers and implications. Best. Pract. Res. Clin. Gastroenterol. 2015, 29, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Bottner, M.; Wedel, T.; Schuppan, D. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated With Immunoglobulin E. Gastroenterology 2019, 157, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Bottner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Luken, J.R.; Gurung, P.; Vogel, P.; Johnson, G.R.; Carter, R.A.; McGoldrick, D.J.; Bandi, S.R.; Calabrese, C.R.; Walle, L.V.; Lamkanfi, M. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 2014, 516, 246–249. [Google Scholar] [CrossRef] [Green Version]
- Pickert, G.; Wirtz, S.; Matzner, J.; AshfaqKhan, M.; Heck, R.; Rosigkeit, S.; Thies, D.; Surabattula, R.; Ehmann, D.; Wehkamp, J.; et al. Wheat consumption aggravates experimental colitis by amylase trypsin inhibitor (ATI)-mediated dysbiosis. Gastroenterology 2020, in press. [Google Scholar] [CrossRef]
- Ibrahim, J.N.; Jounblat, R.; Delwail, A.; Abou-Ghoch, J.; Salem, N.; Chouery, E.; Megarbane, A.; Medlej-Hashim, M.; Lecron, J.C. Ex vivo PBMC cytokine profile in familial Mediterranean fever patients: Involvement of IL-1β, IL-1α and Th17-associated cytokines and decrease of Th1 and Th2 cytokines. Cytokine 2014, 69, 248–254. [Google Scholar] [CrossRef]




| sCD14 (pg/ml) | CRP (mg/L) | SAA (mg/L) | |
|---|---|---|---|
| FMF at baseline | 11357 (9215–14210) | 2.6 (2–9) | 6.9 (0.7–26.7) |
| FMF after wheat challenge | 10023 (9112–1436) | 5.0 (2–9) | 17.3 (2.9–42.7) |
| FMF after placebo (rice) challenge | 11035 (9068–13210) | 3.6 (2–5) | 12.1 (1.8–27,6) |
| NCWS patients (on wheat) | 11089 (9043–12245) | 2.9 (2–7) | 6.7 (1.9–38.4) |
| Healthy controls | 8710 (8023–9205) | 2.6 (1–4) | 4.7 (1.9–34.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carroccio, A.; Mansueto, P.; Soresi, M.; Fayer, F.; Di Liberto, D.; Monguzzi, E.; Lo Pizzo, M.; La Blasca, F.; Geraci, G.; Pecoraro, A.; et al. Wheat Consumption Leads to Immune Activation and Symptom Worsening in Patients with Familial Mediterranean Fever: A Pilot Randomized Trial. Nutrients 2020, 12, 1127. https://doi.org/10.3390/nu12041127
Carroccio A, Mansueto P, Soresi M, Fayer F, Di Liberto D, Monguzzi E, Lo Pizzo M, La Blasca F, Geraci G, Pecoraro A, et al. Wheat Consumption Leads to Immune Activation and Symptom Worsening in Patients with Familial Mediterranean Fever: A Pilot Randomized Trial. Nutrients. 2020; 12(4):1127. https://doi.org/10.3390/nu12041127
Chicago/Turabian StyleCarroccio, Antonio, Pasquale Mansueto, Maurizio Soresi, Francesca Fayer, Diana Di Liberto, Erika Monguzzi, Marianna Lo Pizzo, Francesco La Blasca, Girolamo Geraci, Alice Pecoraro, and et al. 2020. "Wheat Consumption Leads to Immune Activation and Symptom Worsening in Patients with Familial Mediterranean Fever: A Pilot Randomized Trial" Nutrients 12, no. 4: 1127. https://doi.org/10.3390/nu12041127
APA StyleCarroccio, A., Mansueto, P., Soresi, M., Fayer, F., Di Liberto, D., Monguzzi, E., Lo Pizzo, M., La Blasca, F., Geraci, G., Pecoraro, A., Dieli, F., & Schuppan, D. (2020). Wheat Consumption Leads to Immune Activation and Symptom Worsening in Patients with Familial Mediterranean Fever: A Pilot Randomized Trial. Nutrients, 12(4), 1127. https://doi.org/10.3390/nu12041127