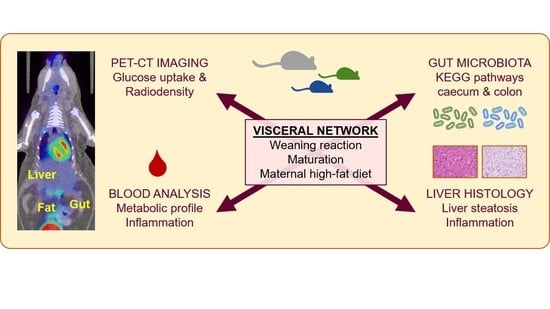
Maturation of the Visceral (Gut-Adipose-Liver) Network in Response to the Weaning Reaction versus Adult Age and Impact of Maternal High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Tissue Metabolism
2.3. Biochemical Analyses
2.4. Liver Histology
2.5. Gut Bacteria 16S rRNA Gene Sequencing
2.6. Statistical Analysis
3. Results
3.1. Effect of Age on Systemic and Tissue Metabolism
3.2. Effect of Maternal Diet on Systemic and Tissue Metabolism
3.3. Tissue Metabolism Associates with Systemic Inflammation, Liver Steatosis, and Steatohepatitis
3.4. Microbiota and Metabolic Pathway Analyses
3.4.1. Effect of Age
3.4.2. Effect of Maternal Diet
4. Discussion
4.1. Effects of Age: Maturation of the Visceral Network
4.2. Effects of Maternal Diet*Age on the Visceral Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 11–20. [Google Scholar] [CrossRef]
- Sanguinetti, E.; Guzzardi, M.A.; Tripodi, M.; Panetta, D.; Selma-Royo, M.; Zega, A.; Telleschi, M.; Collado, M.C.; Iozzo, P. Mi-crobiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Sci. Rep. 2019, 9, 12609. [Google Scholar] [CrossRef] [Green Version]
- Bringhenti, I.; Ornellas, F.; Martins, M.A.; Mandarim-De-Lacerda, C.; Aguila, M.B. Early hepatic insult in the offspring of obese maternal mice. Nutr. Res. 2015, 35, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.; Ethirajan, P.L.; Burdge, G.C.; Bateman, A.C.; Clough, G.F.; Poston, L.; Hanson, M.A.; et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 2009, 50, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Perez, G.; dos Santos, L.S.; dos Santos Cordeiro, G.; Paraguassú, G.M.; Athanazio, D.A.; Couto, R.D.; de Jesus Deiró, T.C.B.; de Castro, R.M.; Barreto-Medeiros, J.M. Maternal and post-weaning exposure to a high fat diet promotes visceral obesity and hepatic steatosis in adult rats. Nutr. Hosp. 2015, 32, 1653–1658. [Google Scholar] [PubMed]
- Kačarević, P.; Grgić, A.; Šnajder, D.; Bijelić, N.; Belovari, T.; Cvijanović, O.; Blažičević, V.; Radić, R. Different combinations of maternal and postnatal diet are reflected in changes of hepatic parenchyma and hepatic TNF-alpha expression in male rat offspring. Acta Histochem. 2017, 119, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidi-ties in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Mitchell, A.; Holmes, A.J.; Denyer, G.S.; Gummesson, A.; Caterson, I.D.; Hunt, N.H.; Storlien, L.H. Role of the Gut in Visceral Fat Inflammation and Metabolic Disorders. Obesity 2011, 19, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic in-flammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [Green Version]
- Van der Poorten, D.; Milner, K.-L.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Kawada, N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhang, X.; Huang, J.; Zeng, Y.; Liu, W.; Geng, C.; Li, K.W.; Yang, D.; Wu, S.; Wei, H.; et al. Relationships between hematopoiesis and hepatogenesis in the midtrimester fetal liver characterized by dynamic tran-scriptomic and proteomic profiles. PLoS ONE 2009, 4, e7641. [Google Scholar] [CrossRef] [Green Version]
- Mandir, N.; FitzGerald, A.J.; Goodlad, R.A. Differences in the effects of age on intestinal proliferation, crypt fission and apop-tosis on the small intestine and the colon of the rat. Int. J. Exp. Pathol. 2005, 86, 125–130. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune sys-tem. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, L.; Lionetti, V.; Burchielli, S.; Simi, C.; Masi, S.; Liistro, T.; Pardini, S.; Porciello, C.; Di Cecco, P.; Vettor, R.; et al. A Dose-Response Elevation in Hepatic Glucose Uptake is Paralleled by Liver Triglyceride Synthesis and Release. Endocr. Res. 2011, 36, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Honka, H.; Makinen, J.; Hannukainen, J.C.; Tarkia, M.; Oikonen, V.; Teräs, M.; Fagerholm, V.; Ishizu, T.; Saraste, A.; Stark, C.; et al. Validation of [18F]fluorodeoxyglucose and positron emission tomography (PET) for the measurement of intestinal metabolism in pigs, and evidence of intestinal insulin resistance in patients with morbid obesity. Diabetologia 2013, 56, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iozzo, P.; Hallsten, K.; Oikonen, V.; Virtanen, K.A.; Parkkola, R.; Kemppainen, J.; Solin, O.; Lonnqvist, F.; Ferrannini, E.; Knuuti, J.; et al. Effects of metformin and rosiglitazone monotherapy on insulin-mediated hepatic glucose uptake and their relation to visceral fat in type 2 diabetes. Diabetes Care 2003, 26, 2069–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, K.A.; Iozzo, P.; Hällsten, K.; Huupponen, R.; Parkkola, R.; Janatuinen, T.; Lönnqvist, F.; Viljanen, T.; Rönnemaa, T.; Lönnroth, P.; et al. Increased Fat Mass Compensates for Insulin Resistance in Abdominal Obesity and Type 2 Diabetes: A Positron-Emitting Tomography Study. Diabetes 2005, 54, 2720–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iozzo, P.; Gastaldelli, A.; Järvisalo, M.J.; Kiss, J.; Borra, R.; Buzzigoli, E.; Viljanen, A.; Naum, G.; Viljanen, T.; Oikonen, V.; et al. 18F-FDG assessment of glucose disposal and production rates during fasting and insulin stimulation: A validation study. J. Nucl. Med. 2006, 47, 1016–1022. [Google Scholar] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Boix-Amoros, A.; Collado, M.C.; Mira, A. Relationship between Milk Microbiota, Bacterial Load, Macronutrients, and Human Cells during Lactation. Front. Microbiol. 2016, 7, 492. [Google Scholar] [CrossRef] [Green Version]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; I Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Rokhsefat, S.; Lin, A.; Comelli, E.M. Mucin–Microbiota Interaction during Postnatal Maturation of the Intestinal Ecosystem: Clinical Implications. Dig. Dis. Sci. 2016, 61, 1473–1486. [Google Scholar] [CrossRef]
- Guarner, V.; Alvarez-Buylla, R. Developmental changes in blood glucose and tissue carbohydrates in the fetal rat: Effects of insulin and adrenaline. Acta Physiol. Pharmacol. Ther. Latinoam. Organo Asoc. Latinoam. Cienc. Fisiol. Asoc. Latinoam. Farm. 1992, 42, 51–59. [Google Scholar]
- Ellsworth, L.; Harman, E.; Padmanabhan, V.; Gregg, B. Lactational programming of glucose homeostasis: A window of oppor-tunity. Reproduction 2018, 156, R23–R42. [Google Scholar] [CrossRef]
- Liu, H.-X.; Rocha, C.S.; Dandekar, S.; Wan, Y.-J.Y. Functional analysis of the relationship between intestinal microbiota and the expression of hepatic genes and pathways during the course of liver regeneration. J. Hepatol. 2015, 64, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Déjardin, F.; Sparwasser, T.; Bérard, M.; Cerf-Bensussan, N.; Eberl, G. Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- AL Nabhani, Z.; Eberl, G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 2020, 13, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornef, M.W.; Torow, N. ‘Layered immunity’ and the ‘neonatal window of opportunity’-timed succession of non-redundant phases to establish mucosal host-microbial homeostasis after birth. Immunology 2020, 159, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Ami-notransferases through MAPK Signaling Pathways in Piglets. Oxidative Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef]
- Mészáros, K.; Lang, C.H.; Bagby, G.J.; Spitzer, J.J. Contribution of different organs to increased glucose consumption after en-dotoxin administration. J. Biol. Chem. 1987, 262, 10965–10970. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kim, H.N.; Chang, Y.; Yun, Y.; Ryu, S.; Shin, H.; Kim, H.L. Gut microbiota and physiologic bowel (18)F-FDG up-take. EJNMMI Res. 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahk, K.; Kim, E.J.; Lee, Y.-J.; Kim, S.; Seo, H.S. Characterization of glucose uptake metabolism in visceral fat by 18 F-FDG PET/CT reflects inflammatory status in metabolic syndrome. PLoS ONE 2020, 15, e0228602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soderborg, T.K.; Clark, S.; Mulligan, C.E.; Janssen, R.C.; Babcock, L.; Ir, D.; Young, B.; Krebs, N.; Lemas, D.J.; Johnson, L.K.; et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Chen, Y.; Zhao, J.; Tang, C.; Jiang, Z.; Geng, B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem. Biophys. Res. Commun. 2009, 380, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Untereiner, A.; Wu, L.; Yang, G. H2S-induced S-sulfhydration of pyruvate carboxylase contributes to gluconeogenesis in liver cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Sterbini, F.P.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2018, 69, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lv, L.; Shi, D.; Ye, J.; Fang, D.; Guo, F.; Li, Y.; He, X.; Li, L. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Front. Microbiol. 2017, 8, 1804. [Google Scholar] [CrossRef]
- Fang, D.; Shi, D.; Lv, L.; Gu, S.; Wu, W.; Chen, Y.; Guo, J.; Li, A.; Hu, X.; Guo, F.; et al. Bifidobacterium pseudocatenulatum LI09 and Bifidobacterium catenulatum LI10 attenuate D-galactosamine-induced liver injury by modifying the gut microbiota. Sci. Rep. 2017, 7, 8770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Yu, L.-X.; Yang, W.; Tang, L.; Lin, Y.; Wu, H.; Zhai, B.; Tan, Y.-X.; Shan, L.; Liu, Q.; et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J. Hepatol. 2012, 57, 803–812. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepato-cellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Liu, P.; Wei, R.; Chen, W.; Rajani, C.; Hernandez, B.Y.; Alegado, R.; Dong, B.; Li, D.; et al. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget 2016, 7, 19355–19366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.E.; Dobrinskikh, E.; Alfonso-Garcia, A.; Fast, A.; Janssen, R.C.; Soderborg, T.K.; Anderson, A.L.; Reisz, J.A.; D’Alessandro, A.; Frank, D.N.; et al. Pyrroloquinoline quinone prevents developmental programming of microbial dysbiosis and macrophage polarization to attenuate liver fibrosis in offspring of obese mice. Hepatol. Commun. 2018, 2, 313–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobhonslidsuk, A.; Chanprasertyothin, S.; Pongrujikorn, T.; Kaewduang, P.; Promson, K.; Petraksa, S.; Ongphiphadhanakul, B. The Association of Gut Microbiota with Nonalcoholic Steatohepatitis in Thais. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.; Kim, H.-N.; Lee, E.-J.; Ryu, S.; Chang, Y.; Shin, H.; Kim, H.-L.; Kim, T.H.; Yoo, K.; Kim, H.Y. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS ONE 2019, 14, e0213692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loubinoux, J.; Bronowickiv, J.P.; Pereira, I.A.; Mougenel, J.L.; Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host re-sistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, P.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; Jiao, J.; Zhang, Y. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Øvrebø, B.; Haj-Yasein, N.; Lee, S.; Svendsen, K.; Hjorth, M.; Bastani, N.E.; Norheim, F.; Drevon, C.A.; Refsum, H.; et al. Effects of dietary methionine and cysteine restriction on plasma biomarkers, serum fibroblast growth factor 21, and adipose tissue gene expression in women with overweight or obesity: A double-blind randomized controlled pilot study. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.X.; Buddha, H.; Castelino-Prabhu, S.; Zhang, Z.; Britton, R.S.; Bacon, B.R.; Neuschwander-Tetri, B.A. Activation of In-sulin-PI3K/Akt-p70S6K Pathway in Hepatic Stellate Cells Contributes to Fibrosis in Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2017, 62, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Z.; Kong, D.; Zhang, X.; Chen, L.; Zhu, X.; Lu, Y.; Zheng, S. Tetramethylpyrazine reduces glucose and insu-lin-induced activation of hepatic stellate cells by inhibiting insulin receptor-mediated PI3K/AKT and ERK pathways. Mol. Cell. Endocrinol. 2014, 382, 197–204. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzardi, M.A.; La Rosa, F.; Campani, D.; Cacciato Insilla, A.; De Sena, V.; Panetta, D.; Brunetto, M.R.; Bonino, F.; Collado, M.C.; Iozzo, P. Maturation of the Visceral (Gut-Adipose-Liver) Network in Response to the Weaning Reaction versus Adult Age and Impact of Maternal High-Fat Diet. Nutrients 2021, 13, 3438. https://doi.org/10.3390/nu13103438
Guzzardi MA, La Rosa F, Campani D, Cacciato Insilla A, De Sena V, Panetta D, Brunetto MR, Bonino F, Collado MC, Iozzo P. Maturation of the Visceral (Gut-Adipose-Liver) Network in Response to the Weaning Reaction versus Adult Age and Impact of Maternal High-Fat Diet. Nutrients. 2021; 13(10):3438. https://doi.org/10.3390/nu13103438
Chicago/Turabian StyleGuzzardi, Maria Angela, Federica La Rosa, Daniela Campani, Andrea Cacciato Insilla, Vincenzo De Sena, Daniele Panetta, Maurizia Rossana Brunetto, Ferruccio Bonino, Maria Carmen Collado, and Patricia Iozzo. 2021. "Maturation of the Visceral (Gut-Adipose-Liver) Network in Response to the Weaning Reaction versus Adult Age and Impact of Maternal High-Fat Diet" Nutrients 13, no. 10: 3438. https://doi.org/10.3390/nu13103438
APA StyleGuzzardi, M. A., La Rosa, F., Campani, D., Cacciato Insilla, A., De Sena, V., Panetta, D., Brunetto, M. R., Bonino, F., Collado, M. C., & Iozzo, P. (2021). Maturation of the Visceral (Gut-Adipose-Liver) Network in Response to the Weaning Reaction versus Adult Age and Impact of Maternal High-Fat Diet. Nutrients, 13(10), 3438. https://doi.org/10.3390/nu13103438