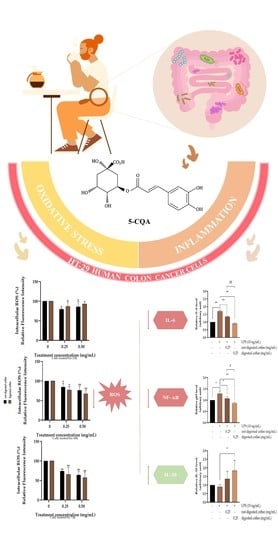
Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling and Coffee Brew Preparation
2.3. UHPLC and Orbitrap HRMS Analysis
2.4. Simulated GiD
2.5. In Vitro Antioxidant Activity
2.5.1. FRAP Assay
2.5.2. ABTS Assay
2.5.3. DPPH Assay
2.6. Total Phenolic Content Assay
2.7. Cell Culture
2.8. Cell Treatment
2.9. Analysis of Cell Viability
2.10. Evaluation of Intracellular ROS Level
2.11. Protein Extraction and Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Identification of Polyphenol Compounds and Caffeine in the Assayed Samples Using UHPLC-Q-Exactive Orbitrap
3.2. Quantification of Polyphenol Compounds and Caffeine in the Assayed Samples Using UHPLC-Q-Exactive Orbitrap
3.3. Antioxidant Activity and Total Phenolic Content
3.4. Effect of not-Digested or Digested Coffee on Cell Viability in HT-29 Cells
3.5. Evaluation of Intracellular ROS Level
3.6. Anti-Inflammatory Effects of Not-Digested and Digested Coffee
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef]
- Lim, D.; Chang, J.; Ahn, J.; Kim, J. Conflicting effects of coffee consumption on cardiovascular diseases: Does coffee consumption aggravate pre-existing risk factors? Processes 2020, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Granby, K.; Fagt, S. Analysis of acrylamide in coffee and dietary exposure to acrylamide from coffee. Anal. Chim. Acta 2004, 520, 177–182. [Google Scholar] [CrossRef]
- Cano-Marquina, A.; Tarín, J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Mure, K.; Maeda, S.; Mukoubayashi, C.; Mugitani, K.; Iwane, M.; Kinoshita, F.; Mohara, O.; Takeshita, T. Habitual coffee consumption inversely associated with metabolic syndrome-related biomarkers involving adiponectin. Nutrition 2013, 29, 982–987. [Google Scholar] [CrossRef]
- Andersen, L.F.; Jacobs, D.R., Jr.; Carlsen, M.H.; Blomhoff, R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2006, 83, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Quesada, C.; Romanos-Nanclares, A.; Navarro, A.M.; Gea, A.; Cervantes, S.; Martínez-González, M.Á.; Toledo, E. Coffee consumption and breast cancer risk in the SUN project. Eur. J. Nutr. 2020, 59, 3461–3471. [Google Scholar] [CrossRef]
- Di Matteo, G.; Spano, M.; Grosso, M.; Salvo, A.; Ingallina, C.; Russo, M.; Ritieni, A.; Mannina, L. Food and COVID-19: Preventive/co-therapeutic strategies explored by current clinical trials and in silico studies. Foods 2020, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Schmit, S.L.; Rennert, H.S.; Rennert, G.; Gruber, S.B. Coffee consumption and the risk of colorectal cancer. Cancer Epidemiol. Prev. Biomark. 2016, 25, 634–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojica, B.E.; Fong, L.E.; Biju, D.; Muharram, A.; Davis, I.M.; Vela, K.O.; Rios, D.; Osorio-Camacena, E.; Kaur, B.; Rojas, S.M. The impact of the roast levels of coffee extracts on their potential anticancer activities. J. Food Sci. 2018, 83, 1125–1130. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.-J.; Heo, Y.-S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis 2011, 32, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Abraham, S. Inhibition of in vivo genotoxicity by coffee. Food Chem. Toxicol. 1989, 27, 787–792. [Google Scholar] [CrossRef]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anti-Cancer Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sarriá, B.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Long-term consumption of a green/roasted coffee blend positively affects glucose metabolism and insulin resistance in humans. Food Res. Int. 2016, 89, 1023–1028. [Google Scholar] [CrossRef]
- Jia, H.; Aw, W.; Egashira, K.; Takahashi, S.; Aoyama, S.; Saito, K.; Kishimoto, Y.; Kato, H. Coffee intake mitigated inflammation and obesity-induced insulin resistance in skeletal muscle of high-fat diet-induced obese mice. Genes Nutr. 2014, 9, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebello, S.A.; Chen, C.H.; Naidoo, N.; Xu, W.; Lee, J.; Chia, K.S.; Tai, E.S.; van Dam, R.M. Coffee and tea consumption in relation to inflammation and basal glucose metabolism in a multi-ethnic Asian population: A cross-sectional study. Nutr. J. 2011, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Giovannucci, E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am. J. Clin. Nutr. 2007, 86, 836S–842S. [Google Scholar] [CrossRef] [Green Version]
- Galeone, C.; Turati, F.; La Vecchia, C.; Tavani, A. Coffee consumption and risk of colorectal cancer: A meta-analysis of case–control studies. Cancer Causes Control. 2010, 21, 1949–1959. [Google Scholar] [CrossRef]
- Gan, Y.; Wu, J.; Zhang, S.; Li, L.; Cao, S.; Mkandawire, N.; Ji, K.; Herath, C.; Gao, C.; Xu, H. Association of coffee consumption with risk of colorectal cancer: A meta-analysis of prospective cohort studies. Oncotarget 2017, 8, 18699. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.J.; Daniel, C.R.; Graubard, B.I.; Wu, J.W.; Hollenbeck, A.R.; Gunter, M.J.; Park, Y.; Freedman, N.D. Caffeinated and decaffeinated coffee and tea intakes and risk of colorectal cancer in a large prospective study. Am. J. Clin. Nutr. 2012, 96, 374–381. [Google Scholar] [CrossRef] [Green Version]
- De Marco, L.M.; Fischer, S.; Henle, T. High molecular weight coffee melanoidins are inhibitors for matrix metalloproteases. J. Agric. Food Chem. 2011, 59, 11417–11423. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Moreira, A.S.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and hydroxycinnamate contents of whole coffee fruits from China, India, and Mexico. J. Agric. Food Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, L.; Lombardi, S.; Gaspari, A.; Rubino, M.; Izzo, L.; Narváez, A.; Ritieni, A.; Grosso, M. In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Spent Coffee Grounds-Enriched Cookies. Foods 2021, 10, 1837. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Arriaga, A.M.; Oomah, B.D.; Campos-Vega, R. Microbiota source impact in vitro metabolite colonic production and anti-proliferative effect of spent coffee grounds on human colon cancer cells (HT-29). Food Res. Int. 2017, 97, 191–198. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Stiebitz, H.; Bytof, G.; Lantz, I.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol. Nutr. Food Res. 2010, 54, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, L.; Izzo, L.; Narváez, A.; Rodríguez-Carrasco, Y.; Grosso, M.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of Different Coffee Brews Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Foods 2021, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Letters 1999, 458, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Vitaglione, P.; Fogliano, V.; Pellegrini, N. Coffee, colon function and colorectal cancer. Food Funct. 2012, 3, 916–922. [Google Scholar] [CrossRef]
- Aljahdali, N.; Gadonna-Widehem, P.; Anton, P.M.; Carbonero, F. Gut Microbiota Modulation by Dietary Barley Malt Melanoidins. Nutrients 2020, 12, 241. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.; Rodríguez-Carrasco, Y.; Pacifico, S.; Castaldo, L.; Narváez, A.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants 2020, 9, 955. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Nutraceutical Fennel Waste Extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A.J. An Environmentally Friendly Practice Used in Olive Cultivation Capable of Increasing Commercial Interest in Waste Products from Oil Processing. Antioxidants 2020, 9, 466. [Google Scholar] [CrossRef]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. Effects of Trichoderma biostimulation on the phenolic profile of extra-virgin olive oil and olive oil by-products. Antioxidants 2020, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical Analysis of Minor Bioactive Components and Cannabidiolic Acid in Commercial Hemp Seed Oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; Grosso, M.; Palumbo, I.; Pesce, M.; D’Alessandro, A.; Zaninotto, G.; Annese, V.; Petruzzelli, R.; Izzo, P.; Sepulveres, R. Allele-specific transcriptional activity of the variable number of tandem repeats of the inducible nitric oxide synthase gene is associated with idiopathic achalasia. United Eur. Gastroenterol. J. 2017, 5, 200–207. [Google Scholar] [CrossRef]
- Riccio, P.; Sessa, R.; de Nicola, S.; Petruzziello, F.; Trombetti, S.; Menna, G.; Pepe, G.; Maddalena, P.; Izzo, P.; Grosso, M. GATA-1 isoforms differently contribute to the production and compartmentation of reactive oxygen species in the myeloid leukemia cell line K562. J. Cell. Physiol. 2019, 234, 20829–20846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodaro, G.; Blasio, G.; Fiorentino, F.; Auberger, P.; Costanzo, P.; Cesaro, E. ZNF224 is a transcriptional repressor of AXL in chronic myeloid leukemia cells. Biochimie 2018, 154, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Voloboueva, L.A.; Liu, J.; Suh, J.H.; Ames, B.N.; Miller, S.S. (R)-α-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4302–4310. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Xu, W.; Wang, J.; Yan, J.; Shi, Y.; Zhang, C.; Ge, W.; Wu, J.; Du, P.; Chen, Y. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 2018, 35, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Qin, L.; Liu, B.; Liu, Y.; Wilson, B.; Eling, T.; Langenbach, R.; Taniura, S.; Hong, J. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J. Neurochem. 2004, 2, 939–947. [Google Scholar] [CrossRef]
- Hsu, H.; Wen, M. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [Green Version]
- Brinkhoff, A.; Sieberichs, A.; Engler, H.; Dolff, S.; Benson, S.; Korth, J.; Schedlowski, M.; Kribben, A.; Witzke, O.; Wilde, B. Pro-inflammatory Th1 and Th17 cells are suppressed during human experimental endotoxemia whereas anti-inflammatory IL-10 producing T-cells are unaffected. Front. Immunol. 2018, 9, 1133. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-S.; Shin, J.-S.; Jang, D.S.; Lee, K.-T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Shi, J.; Shan, S.; Li, H.; Song, G.; Li, Z. Anti-inflammatory effects of millet bran derived-bound polyphenols in LPS-induced HT-29 cell via ROS/miR-149/Akt/NF-κB signaling pathway. Oncotarget 2017, 8, 74582. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, N.; Borde, A.; Wölfel, S.; Åkerman, B.; Larsson, A. Quantification of protein concentration by the Bradford method in the presence of pharmaceutical polymers. Anal. Biochem. 2011, 411, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Becker, M.F.; Keto, J.W.; Kovar, D. Annealing of nanostructured silver films produced by supersonic deposition of nanoparticles. J. Appl. Phys. 2007, 102, 054308. [Google Scholar] [CrossRef]
- Busiello, T.; Ciano, M.; Romano, S.; Sodaro, G.; Garofalo, O.; Bruzzese, D.; Simeone, L.; Chiurazzi, F.; Fiammetta Romano, M.; Costanzo, P. Role of ZNF224 in cell growth and chemoresistance of chronic lymphocitic leukemia. Hum. Mol. Genet. 2017, 26, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Daliu, P.; Narciso, V.; Tenore, G.C.; Novellino, E. Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 2018, 10, 1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eker, M.E.; Karakaya, S. Influence of the addition of chia seeds and germinated seeds and sprouts on the nutritional and beneficial properties of yogurt. Int. J. Gastron. Food Sci. 2020, 22, 100276. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 2018, 245, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Ferron, L.; Frosi, I.; Papetti, A. Advances in static in vitro digestion models after COST action Infogest consensus protocol. Food Funct. 2021, 12, 7619. [Google Scholar] [CrossRef]
- Farah, A. Coffee: Consumption and Health Implications; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Bekedam, E.K.; Schols, H.A.; Van Boekel, M.A.; Smit, G. Incorporation of chlorogenic acids in coffee brew melanoidins. J. Agric. Food Chem. 2008, 56, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Mehta, T.; Esteban-Muñoz, A.; Pastoriza, S.; Paliy, O.; Rufián-Henares, J.Á. Effect of in vitro digestion-fermentation on green and roasted coffee bioactivity: The role of the gut microbiota. Food Chem. 2019, 279, 252–259. [Google Scholar] [CrossRef]
- Goya, L.; Delgado-Andrade, C.; Rufián-Henares, J.A.; Bravo, L.; Morales, F. Effect of coffee melanoidin on human hepatoma HepG2 cells. Protection against oxidative stress induced by tert-butylhydroperoxide. Mol. Nutr. Food Res. 2007, 51, 536–545. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Paz de Peña, M.; Concepción, C.; Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Maalik, A.; Bukhari, S.M.; Zaidi, A.; Shah, K.H.; Khan, F.A. Chlorogenic acid: A pharmacologically potent molecule. Acta Pol. Pharm. 2016, 73, 851–854. [Google Scholar]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef]
- Cornelis, M.C.; El-Sohemy, A. Coffee, caffeine, and coronary heart disease. Curr. Opin. Lipidol. 2007, 18, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Trombetti, S.; Cesaro, E.; Catapano, R.; Sessa, R.; Lo Bianco, A.; Izzo, P.; Grosso, M. Oxidative Stress and ROS-Mediated Signaling in Leukemia: Novel Promising Perspectives to Eradicate Chemoresistant Cells in Myeloid Leukemia. Int. J. Mol. Sci. 2021, 22, 2470. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J. The cytotoxicity of kahweol in HT-29 human colorectal cancer cells is mediated by apoptosis and suppression of heat shock protein 70 expression. Biomol. Ther. 2015, 23, 128. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.-H.; Ko, J.-A.; Jun, W.; Wee, Y.-J.; Walsh, M.K.; Yang, K.-Y.; Choi, J.-H.; Eun, J.-B.; Choi, J.; Kim, Y.-M. Enzymatic synthesis of chlorogenic acid glucoside using dextransucrase and its physical and functional properties. Enzym. Microb. Technol. 2017, 107, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]




| Compound | Chemical | Adduct | RT | Measured | Theoretical | Accuracy |
|---|---|---|---|---|---|---|
| Formula | Ion | (min) | Mass (m/z) | Mass (m/z) | (Δ mg/kg) | |
| Quinic Acid | C7H12O6 | [M−H]− | 1.12 | 191.05531 | 191.05611 | −4.19 |
| 5-CQA | C16H18O9 | [M−H]− | 3.18 | 353.0879 | 353.08780 | 0.03 |
| 4-CQA | C16H18O9 | [M−H]− | 3.19 | 353.08768 | 353.08780 | −0.34 |
| Caffeic Acid | C9H8O4 | [M−H]− | 3.20 | 179.03442 | 179.03498 | −3.13 |
| Caffeine | C8H10N4O2 | [M+H]+ | 3.21 | 195.08757 | 195.08765 | −0.41 |
| 3-CQA | C16H18O9 | [M−H]− | 3.22 | 353.08762 | 353.08780 | −0.51 |
| 3-pCoQA | C16H18O8 | [M−H]− | 3.31 | 337.09232 | 337.09289 | −1.69 |
| 5-pCoQA | C16H18O8 | [M−H]− | 3.32 | 337.0929 | 337.09289 | 0.03 |
| 3-FQA | C17H20O9 | [M−H]− | 3.39 | 367.10309 | 367.10346 | −1.01 |
| 4+5-FQA | C17H20O9 | [M−H]− | 3.40 | 367.10303 | 367.10346 | −1.17 |
| 4,5-CFQA | C26H26O12 | [M−H]− | 3.43 | 529.13495 | 529.13245 | 4.72 |
| Ferulic Acid | C10H10O4 | [M−H]− | 3.46 | 193.05017 | 193.05063 | −2.38 |
| p-Coumaric acid | C9H8O3 | [M−H]− | 3.48 | 163.03934 | 163.04006 | −4.42 |
| 3,4-diCQA | C25H24O12 | [M−H]− | 3.50 | 515.12103 | 515.11950 | 2.97 |
| 3,5-diCQA | C25H24O12 | [M−H]− | 3.53 | 515.11993 | 515.11950 | 0.83 |
| 3,4-FCQA | C26H26O12 | [M−H]− | 3.65 | 529.13247 | 529.13245 | 0.04 |
| Compounds | Not-Digested Coffee Brew | Digested Coffee Brew | ||
|---|---|---|---|---|
| mg/g | SD | mg/g | SD | |
| 3-CQA | 3.40 * | 0.43 | 4.97 * | 0.69 |
| 4-CQA | 5.05 * | 0.05 | 6.92 * | 0.06 |
| 5-CQA | 21.40 * | 0.43 | 25.97 * | 0.69 |
| 3-CoQA | 6.54 | 0.51 | 6.27 | 0.28 |
| 5-CoQA | 7.19 | 0.25 | 7.53 | 0.96 |
| 3-FQA | 9.21 * | 0.38 | 10.56 * | 0.44 |
| 4+5-FQA | 11.90 * | 0.52 | 14.28 * | 0.53 |
| 3,4-diCQA | 1.70 | 0.04 | 1.88 | 0.06 |
| 3,5-diCQA | 0.53 * | 0.02 | 0.74 * | 0.01 |
| 3-FCQA | 1.17 * | 0.05 | 1.41 * | 0.01 |
| 4-CFQA | 0.83 * | 0.02 | 0.98 * | 0.01 |
| TOTAL CGA | 68.92 * | 81.50 * | ||
| Caffeic acid | 0.19 * | 0.01 | 1.55 * | 0.03 |
| Quinic acid | 1.69 * | 0.20 | 2.79 * | 0.02 |
| Ferulic acid | 2.71 * | 0.08 | 4.12 * | 0.07 |
| p-coumaric acid | 0.71 * | 0.08 | 1.12 * | 0.07 |
| TOTAL phenolic | 5.29 * | 9.57 * | ||
| Caffeine | 19.96 * | 0.07 | 17.68 * | 0.27 |
| Samples | FRAP | DPPH | ABTS | TPC | ||||
|---|---|---|---|---|---|---|---|---|
| mmol TE/100 g | ±SD | mmol TE/100 g | ±SD | mmol TE/100 g | ±SD | mg GAE/g | ±SD | |
| Coffee Brew | 67.1 * | 3.2 | 22.8 * | 0.6 | 44.6 * | 1.2 | 97.07 * | 1.52 |
| Coffee Brew Digested | 72.5 * | 0.7 | 51.4 * | 0.3 | 89.2 * | 0.6 | 106.64 * | 2.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients 2021, 13, 4368. https://doi.org/10.3390/nu13124368
Castaldo L, Toriello M, Sessa R, Izzo L, Lombardi S, Narváez A, Ritieni A, Grosso M. Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients. 2021; 13(12):4368. https://doi.org/10.3390/nu13124368
Chicago/Turabian StyleCastaldo, Luigi, Marianna Toriello, Raffaele Sessa, Luana Izzo, Sonia Lombardi, Alfonso Narváez, Alberto Ritieni, and Michela Grosso. 2021. "Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion" Nutrients 13, no. 12: 4368. https://doi.org/10.3390/nu13124368
APA StyleCastaldo, L., Toriello, M., Sessa, R., Izzo, L., Lombardi, S., Narváez, A., Ritieni, A., & Grosso, M. (2021). Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients, 13(12), 4368. https://doi.org/10.3390/nu13124368