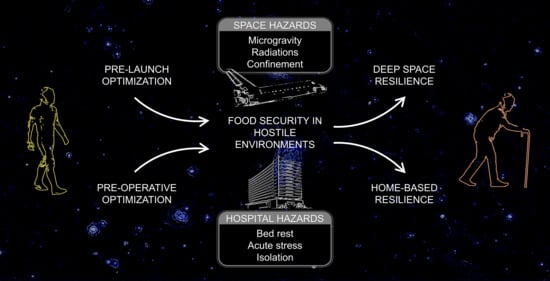
Nutritional Orthopedics and Space Nutrition as Two Sides of the Same Coin: A Scoping Review
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Pre-Operative (Home-Based) vs. Pre-Launch (Earth-Based) Nutritional Issues
3.1.1. Nutritional Program Prior to Elective Orthopedic Surgery
3.1.2. Nutritional Conditioning Prior to Launch and “Packed Launch”
3.2. Post-Operative (Hospital-Based) vs. Space Station (Planetary) Nutritional Issues
3.2.1. Hospital Food Systems: Every Day Your Food Tray
- “Cook and Chill Systems”: outsourced catering systems that prepare, pack, and ship single-serving meals to the hospital, which is therefore equipped with a minimal-functionality kitchen.
- “Cook and Serve Systems”: in-house fully operational kitchen that processes and serves the meals directly to the wards.
3.2.2. Food Research Laboratory in Hospital
3.2.3. Space Food Systems: Where the Light Source Is, the “Onion Spur” Grows
- “Transit Food Systems”: on-orbit food systems in microgravity are based on groundbreaking operations that include prepackaged supplies and low-volume storage.
- “Planetary Surface Food Systems”: partial gravity systems, such as those that are built on other planets, sustain Earth-like processes and include plant crops and animal farms.
3.2.4. Food Research Laboratory in Space
3.3. Hospital vs. Deep Space Hazards and Illnesses
3.3.1. Bedrest, Acute Stress, and Isolation
- Bedrest. When the body is lying down, upright valves of the vascular system do not minimize the gravity-associated fluid shifts. The plasma volume decreases and affects circulation [96], increasing the risk of syncope-related falls [97]. Prolonged immobility accelerates bone and muscle loss, exposing to osteosarcopenia and increased risk of fall-related fractures [97,98]. Immobility is also associated with gastrointestinal affections, such as gastroesophageal reflux, low appetite, slow peristaltic rate, and constipation [17].
- Acute stress. Surgical incision elicits both local (e.g., tissue injury, inflammation, neuroendocrine activation) and systemic (e.g., stress hormones, altered circadian entrainment) responses [18], leading to a wide range of pathological alterations, such as skeletal muscle protein degradation, illness-promoting dysbiosis, and behavioral/psychological disturbances [99]. Surgical pain and post-operative nausea are known to decrease appetite and nutrient intake, thus further delaying recovery [100,101,102].
- Isolation. The unfamiliar hospital diet and sleep-wake rhythm affect the older adults in hospitals, triggering new biological routines. Limited access to visitors, role changes, and relocation have been known for decades to increase loneliness [103]. The sensorial deprivation due to limited interactions outside of the room exposes the old man to delirium along with age-associated spatial disorientation, disequilibrium, and drug-aggravated cognitive decline [90,104].
3.3.2. Microgravity, Radiations, and Confinement
- Microgravity. The absence of eccentric forces exposes the body to the loss of muscle volume/strength and changes in both the composition of muscle fibers and the capillary network [108]. Along with neuromuscular deconditioning [109], the skeletal involution is the most significant alteration that arises after prolonged habitation in space, mainly deriving from the lack of both mechanical forces and sun exposure. Weightless-related conditions also affect gas exchanges and cause cardiovascular derangements, both being linked to fluid accumulation and irregular perfusion [20,110].
- Radiations. In outer space, it is not possible to grow plants in the sunlight. High-energy heavy-ion charged and solar energetic particles cause genome instabilities, carcinogenesis, tissue degeneration, and neurobehavioral disorders [111]. Unfortunately, radiation-derived oxidative stress is deleterious also for bone stability [112], further aggravating the involution of the skeleton.
- Confinement. Prolonged residence in the spaceship’s enclosed habitat exposes the astronauts to hypobaric/hypoxic situations [113] and cognitive decrements that undermine the wellbeing of the entire crew [114]. Space travelers will live in enclosed spaces with artificial illuminations, but crossing multiple time zones and exposure to different light emissions will change the circadian rhythm. The onset of mood swings and sleep disturbances can be unavoidable, being aggravated by the stressful duties [115].
3.4. Hospital-Associated vs. Space-Associated Deconditioning
3.4.1. Nutritional Resilience in Orthopedic Patients
3.4.2. Nutritional Resilience in Astronauts
4. Discussion
- The environmental risks, such as food insecurity, reduced movement, and stressful situations.
- The negative consequences, comprising malnutrition, osteosarcopenia, and low food intake.
- The three control phases of optimization, food security, and resilience.
- The basic strategies of a balanced diet, supplementation, and engineered food systems.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B

Appendix C

Appendix D

Appendix E
| Areas of Nutritional Interest | Sources of Evidence | Topics of the Documents | Scoped Nutritional Features |
|---|---|---|---|
| Nutritional Orthopedics | |||
| Nutritional program prior to surgery | |||
| [8] | Optimization of vitamin D status and cardiac function after surgery. | Vitamin D | |
| [10] | Perioperative nutritional program to optimize recovery. | Tailored dietary prescriptions Nutritional status | |
| [27] | Iron status optimization and preoperative anemia. | Iron | |
| [29] | Preoperative interventions to reduce recreational substances use. | Cessation/reduction of bad food habits | |
| [33,34] | Malnutritional status, immunocompetence, and inflammation. | Nutrition-derived immunocompetence | |
| [38] | Stress response and preoperative fasting vs. loading. | Food abstention | |
| Hospital food systems | |||
| [45,46] | Attitudes of the healthcare sector, catering, and food systems. | Complex food systems Food safety | |
| [50] | Intake of nutrients from electronic vs. paper meal ordering system. | Demand-driven food system | |
| [51] | Plate waste, food costs, and meal ordering system. | Surplus disposal | |
| Food research laboratory in the hospital | |||
| [7] | Orthopedic surgery and optimized recovery programs. | Food abstention | |
| [10,52,63] | Multidisciplinary nutritional programs and dietary supplements. | Advanced nutrition | |
| [27] | Iron status optimization and preoperative anemia. | Iron | |
| [53] | Geriatric osteosarcopenia and therapeutic interventions. | Calcium Proteins | |
| [54] | Optimization of vitamin D status and inflammation after surgery. | Vitamin D | |
| [55,56] | Diet, nutritional status, and interventions for osteoarthritis. | Musculoskeletal health | |
| [59,60,61] | Aging, hedonism, taste dysfunction, intake, and flavor enhancers. | Flavor/pleasure issues | |
| [62] | Facilitators of food waste, patient’s engagement, and food quality. | Meal quality Reduced food intake Food waste | |
| Hospital hazards | |||
| [90,91] | Multifaceted aspects of geriatric syndrome. | Osteosarcopenia | |
| [92,93] | Mental and physical disabilities associated with hospitalization. | Low mechanical forces | |
| [96] | Inactivity, deterioration, and bed rest. | Fluid shifts Cardiovascular health | |
| [97,98] | Hospital hazards, sarcopenia, and risk of falls. | Protein/bone loss Risk of falls/fracture | |
| [17] | Hospital-associated deconditioning and orthostatic intolerance. | Gastrointestinal health | |
| [18,99] | Acute response to surgery and bacterial overgrowth in critical care. | Protein/bone loss Dysbiosis Neurobehavioral health | |
| [100,101,102] | Pain control, nausea, and early physical recovery. | Reduced food intake Flavor/pleasure issues | |
| [103] | Management of loneliness in hospitals. | Neurobehavioral health | |
| [90,104] | Multifaceted aspects of age-related deteriorations. | Spatial disorientation Proprioception Cognitive decrements | |
| Nutritional resilience in orthopedic patients | |||
| [10] | Perioperative nutritional program for optimizing recovery. | Food insecurity | |
| [33] | Malnutritional status and immunocompetence. | Reduced food supply Food insecurity Flavor/pleasure issues | |
| [53,118] | Geriatric osteosarcopenia and therapeutic interventions. | Vitamin D Calcium Proteins | |
| [90,119] | Multifaceted geriatric syndrome and multimodal interventions. | Meal timing Flavor/pleasure issues | |
| [116] | Age-related anorexia and treatments. | Reduced food intake Flavor/pleasure issues | |
| [117] | β-hydroxy-β-methylbutyrate in older adults. | Advanced nutrition | |
| [120,121,123] | Hospital-associated deconditioning, interventions, and outcomes. | Osteosarcopenia Risk of falls/fracture | |
| Space Nutrition | |||
| Nutritional conditioning prior to launch | |||
| [39] | Food to sustain life during early ventures in space. | Nutritional status | |
| [40] | Immunocompetence, in-flight supplementation, and workout. | Nutrition-derived immunocompetence | |
| [19] | Food, beverage, and menus in space. | Tailored dietary prescriptions | |
| [25] | Aeromedical factors and pilot’s health and performance. | Cessation/reduction of bad food habits | |
| [20] | Astronaut’s health and performance. | Food abstention | |
| [42] | Food and nutrition sciences for exploration missions in space. | Advanced nutrition Food safety | |
| Space food systems | |||
| [21,64,67] | History of space food, system development, technical goals. | Complex food systems | |
| [66,67] | History of space plant growth systems, development trends. | Demand-driven food system Reduced food supply Surplus disposal | |
| [22,68,73] | Challenges for sustainable systems in space, insects as foods. | Advanced nutrition | |
| Food research laboratory in space | |||
| [20] | Astronaut’s health and performance. | Flavor/pleasure issues | |
| [23,64,67] | History of space food, system development, food science goals. | Meal quality Food waste | |
| [76,77] | Food growth and nutrient performance in space. | Advanced nutrition | |
| [79] | Analysis of nutrient contents in processed space foods. | Food insecurity | |
| [82] | Interventions for bone loss during long-term spaceflight. | Vitamin D Calcium Musculoskeletal health | |
| [83,85] | Musculoskeletal involution and other space-related issues. | Proteins Musculoskeletal health | |
| [84] | Microgravity and radiation, musculoskeletal health, iron status. | Iron | |
| Space hazards | |||
| [20,110] | Astronaut’s health, performance, and cardiac function. | Fluid shifts Cardiovascular health | |
| [95] | The role of the constant and pervasive force of gravity. | Low mechanical forces | |
| [87,105,106] | Translation of space research, bodily affections of microgravity. | Proprioception Spatial disorientation | |
| [24,107,108,109] | Musculoskeletal remodeling, space hazards, countermeasures. | Musculoskeletal health | |
| [111] | Biological affections from space radiation exposure. | Neurobehavioral health | |
| [112] | Mechanisms of the oxidative stress-induced involution in space. | Protein/bone loss | |
| [113] | Physiological adjustments to hostile environments. | Reduced food intake | |
| [114] | Dietary intakes, requirements, and psychosocial aspects in space. | Cognitive decrements | |
| [115] | Immune dysfunction, countermeasures for exploration missions. | Neurobehavioral health | |
| Nutritional resilience in space travellers | |||
| [122] | Changes induced by models of microgravity and osteosarcopenia. | Osteosarcopenia | |
| [124] | Comprehensive human experience of eating in space. | Flavor/pleasure issues | |
| [125] | Malnutrition after long-term space flight. | Nutritional statusMeal timingFood insecurity | |
| [127,128,129,130] | Gastrointestinal affections of microgravity and research goals. | Gastrointestinal health Dysbiosis | |
| [133,134] | Performance risks associated with supplements in aviation. | Food safety |
Appendix F
| Areas of Nutritional Interest | Author, Year | Editorial Source, Document Type | Population or Setting/Findings |
|---|---|---|---|
| Nutritional Orthopedics | |||
| Nutritional program prior to surgery | |||
| [8] Briguglio, 2018 | J Geriatr Cardiol, Human study | Orthopedic patients/Vitamin D supplementation may be associated with an amelioration of cardiac function and hemodynamic. | |
| [10] Briguglio, 2019 | Nutr Clin Metab, Review | Orthopedic patients/A nutritional program accounting for preoperative, postoperative, and support after discharge should be integrated. | |
| [27] Briguglio, 2020 | Nutrients, Human study | Orthopedic patients/Oral iron can optimize the preoperative iron status, with gastrointestinal diseases and medications affecting efficacy. | |
| [29] Sarin, 2017 | J Surg Oncol, Review | Hospital environment/Oral clear fluid intake or carbohydrate-based beverages should be liberalized prior to elective surgery. | |
| [33] Budworth, 2019 | Front Psychol, Meta-analysis | Hospital environment/Alcohol or other recreational substance use should be reduced by targeted preoperative interventions. | |
| [34] Durrand, 2019 | Clin Med (Lond), Review | Hospital environment/Prehabilitation principles interest psychology, exercise, nutrition, alcohol, smoking, and patient’s education. | |
| [38] Mau, 2018 | Exp Gerontol, Review | Older adults/Adipose tissue inflammation is associated with impaired metabolic health, energy storage, and lipid metabolism. | |
| Hospital food systems | |||
| [45] Hofer, 2013 | Int J Facil Manag, Public health study | Hospitalized patients/Cost transparency, benchmarking activities, and cost of meals per patient in hospitals need to be examined. | |
| [46] Edwards, 2006 | J Hum Nutr Diet, Human study | Hospitalized patients/Novel food systems may improve food intakes and satisfaction along with reduced wastage at the ward level. | |
| [50] Maunder, 2015 | Clin Nutr ESPEN, Human study | Hospitalized patients/Dietary intake and satisfaction may be enhanced by an electronic meal ordering system compared to a paper menu. | |
| [51] McCray, 2018 | Clin Nutr ESPEN, Human study | Hospitalized patients/Food waste, patient’s engagement, and costs can be controlled by an electronic bedside meal ordering system. | |
| Food research laboratory in hospital | |||
| [7] Kaye, 2019 | J Anaesthesiol Clin Pharmacol, Review | Hospital environment/Enhanced recovery pathways consist of perioperative interventions to improve outcomes and reduce stay. | |
| [10] Briguglio, 2019 | Nutr Clin Metab, Review | Orthopedic patients/A nutritional program accounting for preoperative, postoperative, and support after discharge should be integrated. | |
| [27] Briguglio, 2020 | Nutrients, Human study | Orthopedic patients/Oral iron can optimize the preoperative iron status, with gastrointestinal diseases and medications affecting efficacy. | |
| [52] Rask, 2020 | Mil Med, Public health study | Orthopedic patients/Multivitamin supplementation may enhance the recovery, but dosages and duration should be investigated. | |
| [53] Fatima, 2019 | Adv Musculoskelet Dis, Review | Older adults/Nonpharmacological strategies, such as nutrition and exercise, may be considered for treating osteosarcopenia. | |
| [54] Briguglio, 2020 | Nutr Clin Metab, Human study | Orthopedic patients/The correction of hypovitaminosis D is associated with a reduction of inflammation and endothelial dysfunction. | |
| [55] Purcell, 2016 | Can J Diet Pract Res, Human study | Orthopedic patients/Before and after surgery, targeted dietary and exercise interventions may be useful in patients with osteoarthritis. | |
| [56] Morales-Ivorra, 2018 | Nutrients, Review | Orthopedic patients/There may be an association between the onset of osteoarthritis and the adherence to a Mediterranean dietary pattern. | |
| [59] Joussain, 2013 | PLoS One, Human study | Older adults/Normal aging is associated with reduced odor perception, possibly related to changes in pleasant odor processing. | |
| [60] Iannilli, 2017 | J Neurosci Res, Human study | Older adults/Age-related reduction of taste may be due to both central and peripheral degradation of neural signatures. | |
| [61] Mathey, 2001 | J Geron A Biol Sci Med Sci, Human study | Older adults/Dietary intake and body weight in institutionalized older adults may be improved by adding flavor boosters to foods. | |
| [62] Schiavone, 2019 | Int J Envir Res Pub Health, Human study | Hospitalized patients/Quality improvement of meal reservation, accounting specific preferences, is promising for plate waste reduction. | |
| [63] Bell, 2014 | Clin Nutr, Human study | Orthopedic patients/Multimodal nutritional support plus foodservice enhancement increase food intakes and improve outcomes. | |
| Hospital hazards | |||
| [90] Briguglio, 2020 | Front Psychol, Opinion | Older adults/Home-confinement generates a more fragile class of older adults, with long-term psychological consequences. | |
| [91] Belchior, 2020 | Eur Geriatr Med, Review | Older adults/Early interventions, like resistance exercise and adequate protein, vitamin D, calcium, may delay osteosarcopenia onset. | |
| [92] Covinsky, 2011 | JAMA, Review | Hospital environment/Hospitalization-associated disability may be prevented towards functional status studies in acute geriatric units. | |
| [93] Friedman 2008 | Gerontologist, Human study | Hospitalized patients/Hospitalization is associated with high risks for falls and functional decline. Targeted programs should be developed. | |
| [96] Corcoran, 1991 | West J Med, Review | Hospital environment/Inactivity damages each of the body’s organ systems similarly to aging. Preventive strategies should be planned. | |
| [97] Creditor, 1993 | Ann Intern Med, Review | Hospital environment/De-emphasizing bed rest and actively facilitating ambulation and socialization can avoid the dependency cascade. | |
| [98] Landi, 2012 | Clin Nutr, Human study | Older adults/Sarcopenia is highly prevalent, leading to greater risks for fall, regardless of age, gender, or other confounding factors. | |
| [17] Han, 2018 | Elsevier, Book chapter | Hospitalized patients/Bed rest should be avoided. Early mobilization should be promoted to counteract multiple system dysfunction. | |
| [18] Hashmi, 2018 | Cambridge, Book chapter | Hospitalized patients/The adaptation to stress impairs with aging. Surgical trauma in older adults is associated with impaired recovery. | |
| [99] McDonald, 2016 | mSphere, Human study | Hospitalized patients/Health-promoting microbiome signatures may guide to therapeutic interventions in critical illnesses. | |
| [100] Eriksson, 2017 | J Adv Nurs, Human study | Orthopedic patients/It is critical to monitor a patient’s pain. It is associated with physical recovery after surgery and with general outcomes. | |
| [101] Eriksson, 2019 | J Adv Nurs, Human study | Orthopedic patients/It is important to monitor postoperative nausea. The simple measure of intensity is associated with physical recovery. | |
| [102] Falzone, 2013 | Drugs Aging, Review | Older adults/For most analgesic strategies, “start low and go slow” should be adopted in view of pharmacokinetic-dynamic changes. | |
| [103] Rodgers, 1989 | J Gerontol Nurs, Opinion | Hospitalized patients/Nurses, through ensuring access to visitors and facilitating involvement in activities, may decrease loneliness. | |
| [104] Dunsky, 2019 | Front Aging Neurosci, Review | Older adults/Dual-task function-oriented challenges along with balance control can stimulate the sensory-neuromuscular controls. | |
| Nutritional resilience in orthopedic patients | |||
| [10] Briguglio, 2019 | Nutr Clin Metab, Review | Orthopedic patients/A nutritional program accounting for preoperative, postoperative, and support after discharge should be integrated. | |
| [33] Budworth, 2019 | Front Psychol, Meta-analysis | Hospital environment/Alcohol or other recreational substance use should be reduced by targeted preoperative interventions. | |
| [53] Fatima, 2019 | Adv Musculoskelet Dis, Review | Older adults/Nonpharmacological strategies, such as nutrition and exercise, may be considered for treating osteosarcopenia. | |
| [90] Briguglio, 2020 | Front Psychol, Opinion | Older adults/Home-confinement generates a more fragile class of older adults, with long-term psychological consequences. | |
| [116] Landi, 2016 | Nutrients, Review | Older adults/Age-related anorexia negatively influences appetite and food intake. Oral supplements or modified diets should be studied. | |
| [117] Oktaviana, 2019 | J Nutr Health Aging, Review | Old patients/The impairment of muscle mass and strength in sarcopenia or frailty may be preserved by β-hydroxy-β-methylbutyrate. | |
| [118] Kirk, 2020 | J Cachexia Sarcopenia Muscle, Editorial | Older adults/Osteosarcopenia is an emerging geriatric syndrome and the preventive maximization of musculoskeletal health is mandatory. | |
| [119] Briguglio, 2020 | Neuropsychiatr Dis Treat, Opinion | Older adults/Healthy eating, exercise, and sleep are critical components to prevent and manage neuropsychiatric disorders. | |
| [120] Bae, 2020 | Ger Orthop Surg Rehab, Human study | Orthopedic patients/Osteoporosis is correlated to sarcopenia. Osteosarcopenia is associated with poor outcomes and high fracture rates. | |
| [121] Smith, 2020 | Arch Gerontol Geriatr, Meta-analysis | Hospitalized patients/There is the need for more studies on enhanced programs targeted at hospital-associated deconditioning. | |
| [123] Paintin, 2019 | Br J Hosp Med (Lond), Review | Older adults/Osteosarcopenia is associated with individual falls, fractures, mortality, and socioeconomic burden for the society. | |
| Space Nutrition | |||
| Nutritional conditioning prior to launch | |||
| [39] Unknown, 1960 | Nutr Rev, Review | Space environment/Advanced inactivation methods, packaging, and lightweight food systems are critical points to sustain health in space. | |
| [40] Baba, 2020 | Nutrients, Human study | Healthy individuals/The space diet lacks omega-3 fatty acids, β-alanine, and carnosine. Supplementation should be considered. | |
| [19] Casaburri, 1999 | NASA, Guide | Space environment/In-depth knowledge about food science and technology is required to preserve and package food for space. | |
| [25] FAA, 2016 | FAA, Book chapter | Pilots/Flying requires mental and physical standards to resist hypoxia, spatial disorientation, motion sickness, and other major issues. | |
| [20] Lane, 2010 | NASA, Book chapter | Space environment/Research and health care are critical factors during shuttle flights and missions beyond low-Earth orbit. | |
| [42] Douglas, 2020 | J Nutr, Opinion | Space environment/Space food should meet safety, stability, palatability, variety, reliability, resource minimization, usability, nutrition. | |
| Space food systems | |||
| [64] Perchonok, 2002 | Nutrition, Review | Space environment/Extended space missions need nutritious and easily consumable food along with advanced crops for food security. | |
| [66] Zabel, 2016 | Life Sci Space Res (Amst), Review | Space food systems/Novel agriculture technologies for bio-regenerative life support systems can enable long-term space missions. | |
| [67] Oluwafemi, 2018 | Adv Astronaut Sci Technol, Review | Space environment/Knowledge on plant growth, methods to simulate soils, and greenhouse facilities should be addressed for space. | |
| [21] Massa, 2018 | NASA, Technical report | Vegetable-production system/Advanced food systems can grow fresh vegetables, helping to mitigate the risk of inadequate supplies. | |
| [68] Carillo, 2020 | Agron J, Review | Space food systems/Bio-regenerative life support systems should fulfill physical constraints and energy requirements for space farming. | |
| [22] Dufour, 1981 | NASA, Technical report | Insects/Insects are a potential food source in space, providing a stream of proteins for sustaining astronauts’ health. | |
| [73] Tong, 2011 | Bull Entomol Res, Animal study | Insects/To feed silkworms with inedible biomass is a promising approach for providing high-quality proteins for astronauts. | |
| Food research laboratory in space | |||
| [20] Lane, 2010 | NASA, Book chapter | Space environment/Research and health care are critical factors during shuttle flights and missions beyond low-Earth orbit. | |
| [64] Perchonok, 2002 | Nutrition, Review | Space environment/Extended space missions need nutritious and easily consumable food along with advanced crops for food security. | |
| [67] Oluwafemi, 2018 | Adv Astronaut Sci Technol, Review | Space environment/Knowledge on plant growth, methods to simulate soils, and greenhouse facilities should be addressed for space. | |
| [23] Brief, 2015 | NASA, Online report | Space food systems/Surplus disposal, reduced mass/volume of the food system, and extended shelf life are vital research goals for space. | |
| [76] Khodadad, 2020 | Front Plant Sci, Food study | Lettuce/Leafy vegetables from the Veggie plant growth units can provide fresh and safe food to integrate the astronauts’ diet. | |
| [77] Yuan, 2017 | Aquat Bot, Food study | Duckweed/Simulated microgravity does not significantly affect duckweed growth, ultra-structure, and starch content. | |
| [79] Cooper, 2017 | NPJ Microgravity, Food study | Space food/Space food stored over 3 years encounter nutritional impoverishment. Advanced methods should stabilize nutrient content. | |
| [82] Smith, 2012 | J Bone Miner Res, Human study | Astronauts/Improving nutrition and resistance exercise in space can mitigate the musculoskeletal involution over 6-month missions. | |
| [83] Hackney, 2014 | Life (Basel), Review | Astronauts/To protect musculoskeletal health and to increased protein/amino acid intakes with exercise may be promising. | |
| [84] Yang, 2018 | Int J Mol Sci, Review | Astronauts/Iron overload and oxidative damage are deleterious effects of space. Iron chelators and antioxidants should be investigated. | |
| [85] Stein, 2001 | Nutr Res Rev, Review | Astronauts/Space habitation is associated with musculoskeletal remodeling, protein and bone loss, and reduced dietary intakes. | |
| Space hazards | |||
| [20] Lane, 2010 | NASA, Book chapter | Space environment/Research and health care are critical factors during shuttle flights and missions beyond low-Earth orbit. | |
| [87] Shelhamer, 2020 | NPJ Microgravity, Perspective | Space environment/Findings from space research, like the research results from bone physiology, have enhanced healthcare on Earth. | |
| [95] Page, 1977 | N Engl J Med, Review | Space environment/Physiologic and morphologic changes in animals raised in weightlessness should be a prospect for future research. | |
| [105] Ferrè, 2019 | Sci Rep, Human study | Healthy individuals/The perception of weight depends on immediate sensory signals after an experimentally altered gravitation. | |
| [106] Bizzarri, 2015 | BioMed Research International, Editorial | Space environment/More appropriate experimental models are needed to study the gravity-related changes of cellular dynamics. | |
| [107] Stein, 2013 | Eur J Appl Physiol, Perspective | Space environment/Energy, muscle, bone issues may have been exaggerated. More studies on musculoskeletal involution are needed. | |
| [110] Palacios, 2019 | Front Physiol, Human study | Healthy individuals/Microgravity is associated with an increased risk of repolarization instabilities and arrhythmias. | |
| [24] Abadie, 2020 | NASA, Online document | Astronauts/Space hazards are gravity, isolation, confinement, radiation, and distance. Their effects should be minimized. | |
| [108] Fitts, 2010 | J Physiol, Human study | Astronauts/Prolonged stay in space causes loss of muscle fiber mass, force, and power. Exercise countermeasures should be improved. | |
| [109] English, 2019 | Compr Physiol, Review | Astronauts/It is vital to study physiological thresholds below which task performance is reduced along with exercise countermeasures. | |
| [111] McDonald, 2020 | Cancers (Basel), Bioinformatics study | Animal models/Novel hypotheses on the human risks from space radiations can be generated from the GeneLab’s rich database. | |
| [112] Tian, 2017 | Int J Mol Sci, Review | Space environment/Radiations and microgravity in space can elicit oxidative stress in humans, causing a deleterious skeletal involution. | |
| [113] Pasiakos, 2020 | Annu Rev Nutr, Review | Hostile environment/Extreme temperatures and high-altitude alter expenditures and intakes, not fully met by dietary recommendations. | |
| [114] Lane, 1994 | Am J Clin Nutr, Review | Astronauts/Dietary intakes may be lower than predicted, impacting the endocrine, immune, musculoskeletal, and psychosocial systems. | |
| [115] Crucian, 2018 | Front Immunol, Review | Space environment/It is important to study immune countermeasures in space, which comprise supplements, foods, exercise, or drugs. | |
| Nutritional resilience in space travelers | |||
| [122] Tomilovskaya, 2019 | Front Physiol, Review | Space environment/Dry immersion accurately simulates factors of space flight, reproducing the full spectrum of bodily changes. | |
| [124] Obrist, 2019 | Front Comput Sci, Human/Food study | Healthy individuals/Food has a multi-dimensional and -sensorial role, and the eating experiences in space should account for all these areas. | |
| [125] Smith, 2005 | J Nutr, Human study | Astronauts/After an extended stay in space, nutritional changes occur, comprising bone loss, poor vitamin D status, and oxidative damage. | |
| [127] Amidon, 1991 | J Clin Pharmacol, Review | Space environment/The absence of gravity may negatively affect gastric emptying, intestinal transit, and drug absorption. | |
| [128] Millet, 2001 | Clin Physiol, Human study | Healthy individuals/Controlled physical inactivity during head-down bed rest caused altered sympathetic activity and protein metabolism. | |
| [129] Alvarez, 2019 | Sci Rep, In vitro study | Astronauts/Gastrointestinal homeostasis in space encounters a protracted susceptibility to epithelial barrier disruption. | |
| [130] Bergouignan, 2016 | NPJ Microgravity, Review | Space environment/Space research interests include energy balance, eating, metabolic stress, deficiencies, dysbiosis, and fluid balance. | |
| [133] Barker, 2011 | Aviat Space Environ Med, Case report | Pilots/G tolerance can be altered by dietary supplements in turn potentially causing a catastrophic G-induced loss of consciousness. | |
| [134] Sather, 2013 | The Sport Journal, Review | Pilots/Military aviators may use dietary supplements to enhance performance, but safety and effectiveness should be regulated. |
References
- Hueston, W.; McLeod, A. Overview of the global food system: Changes over time/space and lessons for future food safety. In Improving Food Safety through a One Health Approach; Institute of Medicine, Ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Lippi, G. The central role of iron in human nutrition: From folk to contemporary medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Chappell, C. Grandma’s Remedies: A Guide to Traditional Cures and Treatments from Mustard Poultices to Rosehip Syrup; Cornerstone Digital: Penguin Random House: London, UK, 2009. [Google Scholar]
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satia, J.A. Dietary acculturation and the nutrition transition: An overview. Appl. Physiol. Nutr. Metab. 2010, 35, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Banfi, G.; Vitale, J.; Sirtori, P.; Perazzo, P. The Prototypic Nutritional Assessment and the Parsing of the Nutritional Critical Control Points (NCCP) in Orthopedics; Italian Society of Enteral and Parenteral Nutrition (SINPE): Milan, Italy, 2019. [Google Scholar]
- Kaye, A.D.; Urman, R.D.; Cornett, E.M.; Hart, B.M.; Chami, A.; Gayle, J.A.; Fox, C.J. Enhanced recovery pathways in orthopedic surgery. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S35–S39. [Google Scholar] [CrossRef]
- Briguglio, M.; Gianturco, L.; Stella, D.; Colombo, C.; Bonadies, M.; Sala, O.; Anselmi, M.; Lippi, G.; Turiel, M. Correction of hypovitaminosis D improved global longitudinal strain earlier than left ventricular ejection fraction in cardiovascular older adults after orthopaedic surgery. J. Geriatr. Cardiol. 2018, 15, 519–522. [Google Scholar]
- Briguglio, M.; Porta, M.; Zuffada, F.; Bona, A.R.; Crespi, T.; Pino, F.; Perazzo, P.; Mazzocchi, M.; Giorgino, R.; De Angelis, G.; et al. SARS-CoV-2 aiming for the heart: A multicenter Italian perspective about cardiovascular issues in COVID-19. Front. Physiol. 2020, 11, 571367. [Google Scholar] [CrossRef]
- Briguglio, M.; Gianola, S.; Aguirre, M.-F.I.; Sirtori, P.; Perazzo, P.; Pennestri, F.; Brayda-Bruno, M.; Sansone, V.; Banfi, G. Nutritional support for enhanced recovery programs in orthopedics: Future perspectives for implementing clinical practice. Nutr. Clin. Métabolisme 2019, 33, 190–198. [Google Scholar] [CrossRef]
- Dismukes, K.; Petty, J.I. Food for Space Flight 2004 (Updated on 25 Novemeber 2019). Available online: https://www.nasa.gov/audience/forstudents/postsecondary/features/F_Food_for_Space_Flight.html (accessed on 22 September 2020).
- Bourland, C.T. Food systems for space travel. Life Support Biosph. Sci. 1999, 6, 9–12. [Google Scholar]
- Champlin, G.A.; MC, U. S. Army. The challenge of man’s entry into space. Mil. Med. 1958, 123, 422–427. [Google Scholar] [CrossRef]
- Lane, H.W.; Feeback, D.L. History of nutrition in space flight: Overview. Nutrition 2002, 18, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Ebbs, J.C. Nutrition in the space age. Nutr. Rev. 2009, 17, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Fanzo, J.; Bellows, A.L.; Spiker, M.L.; Thorne-Lyman, A.L.; Bloem, M.W. The importance of food systems and the environment for nutrition. Am. J. Clin. Nutr. 2021, 113, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-S.; Chen, S.-C. Prevention of Hospital-Acquired Deconditioning, in Geriatric Rehabilitation; Elsevier: Missouri, USA, 2018; pp. 111–119. ISBN 9780323544559. [Google Scholar]
- Hashmi, N.K.; Podgoreanu, M.V. Stress Response to Surgery in the Elderly, pp. 168–186 in Principles of Geriatric Critical Care; Cambridge University Press: Cambridge, UK, 2018; ISBN 9781316613894. [Google Scholar]
- Casaburri, A.A.; Gardner, C.A. Space Food and Nutrition—An Educator’s Guide with Activities in Science and Mathematics; National Aeronautics and Space Administration: Washington, DC, USA, 1999. [Google Scholar]
- Lane, H.; Young, L.; Paloski, W.; Barger, L.; Czeisler, C.; Charles, J.F.; Platts, S.; Feeback, D.; Baldwin, K.; Hayes, J.; et al. Astronaut health and performance. In Wings in Orbit: Scientific and Engineering Legacies of the Space Shuttle; Hale, W., Lane, H., National Aeronautics and Space Administration, Lulla, K., Chapline, G., Eds.; National Aeronautics and Space Administration, Government Printing Office: Houston, TX, USA, 2010. [Google Scholar]
- Massa, G.D.; Wheeler, R.M.; Romeyn, M.W.; Hummerick, M.E.; Spencer, L.E.; Morrow, R.C.; Mitchell, C.A.; Burgner, S.; Whitmire, A.M.; Young, M.H.; et al. Preparation for Pick-and-Eat Food Production on the International Space Station: Flight Definition for the VEG-04 and VEG-05 Missions; NASA Technical Reports Server:NASA Human Research Program Investigators Workshop: Galveston, TX, USA, 2018. [Google Scholar]
- Dufour, P.A. Insects: A Nutritional Alternative; Life Sciences Division, National Aeronautics and Space Administration: Washington, DC, USA, 1981. [Google Scholar]
- Brief, J. Space Food Systems, 2015 (Updated on 7 August 2017). Available online: https://www.nasa.gov/content/space-food-systems (accessed on 22 September 2020).
- Abadie, L.J.; Lloyd, C.W.; Shelhamer, M.J. The Human Body in Space 2020. Available online: https://www.nasa.gov/hrp/bodyinspace (accessed on 22 September 2020).
- Federal Aviation Administration. Aeromedical factors. In Pilot’s Handbook of Aeronautical Knowledge; Federal Aviation Administration, Ed.; United States Department of Transportation, Federal Aviation Administration, Airman Testing Standards Branch: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Bardram, L.; Funch-Jensen, P.; Kehlet, H.; Crawford, M. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995, 345, 763–764. [Google Scholar] [CrossRef]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; De Vecchi, E.; Lombardi, G.; Lippi, G.; Riso, P.; Porrini, M.; Sergio, R.; Pino, F.; et al. Oral supplementation with sucrosomial ferric pyrophosphate plus l-ascorbic acid to ameliorate the martial status: A randomized controlled trial. Nutrients 2020, 12, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, R.S.; Tufts, C.W.; DePinto, D.G.; Chen, J.; Altshuler, J.R.; Serdiuk, A.; Cohen, J.B.; Patel, S.Y. How sweet is this? A review and evaluation of preoperative carbohydrate loading in the enhanced recovery after surgery model. Nutr. Clin. Pr. 2020, 35, 246–253. [Google Scholar] [CrossRef]
- Sarin, A.; Chen, L.-L.; Wick, E.C. Enhanced recovery after surgery—Preoperative fasting and glucose loading—A review. J. Surg. Oncol. 2017, 116, 578–582. [Google Scholar] [CrossRef]
- Hellström, P.M.; Samuelsson, B.; Al-Ani, A.N.; Hedström, M. Normal gastric emptying time of a carbohydrate-rich drink in elderly patients with acute hip fracture: A pilot study. BMC Anesthesiol. 2017, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Choi, Y.S.; Shin, H.; Yang, I.H.; Park, K.K.; Kwon, H.M.; Kang, B.; Kim, S.Y. Preoperative carbohydrate drinks do not decrease postoperative nausea and vomiting in type 2 diabetic patients undergoing total knee arthroplasty—A randomized controlled trial. J. Am. Acad. Orthop. Surg. 2021, 29, 35–43. [Google Scholar] [CrossRef]
- Theadom, A.; Cropley, M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: A systematic review. Tob. Control 2006, 15, 352–358. [Google Scholar] [CrossRef]
- Budworth, L.; Prestwich, A.; Lawton, R.; Kotzé, A.; Kellar, I. Preoperative Interventions for alcohol and other recreational substance use: A systematic review and meta-analysis. Front. Psychol. 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Durrand, J.; Singh, S.J.; Danjoux, G. Prehabilitation. Clin. Med. J. 2019, 19, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevåg, O.M.; Jorde, R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur. J. Endocrinol. 2015, 172, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Schafer, L.; Overvad, K. Subcutaneous adipose-tissue fatty acids and vitamin E in humans: Relation to diet and sampling site. Am. J. Clin. Nutr. 1990, 52, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Pregliasco, F.E.; Lombardi, G.; Perazzo, P.; Banfi, G. The malnutritional status of the host as a virulence factor for new coronavirus SARS-CoV-2. Front. Med. 2020, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Mau, T.; Yung, R.L. Adipose tissue inflammation in aging. Exp. Gerontol. 2018, 105, 27–31. [Google Scholar] [CrossRef]
- Nutrition for man in space. Nutr. Rev. 1960, 18, 100–101. [CrossRef]
- Baba, S.; Smith, T.; Hellmann, J.; Bhatnagar, A.; Carter, K.; Vanhoover, A.; Caruso, J. Space flight diet-induced deficiency and response to gravity-free resistive exercise. Nutrients 2020, 12, 2400. [Google Scholar] [CrossRef]
- Verma, M.; Hontecillas, R.; Tubau-Juni, N.; Abedi, V.; Bassaganya-Riera, J. Challenges in personalized nutrition and health. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Douglas, G.L.; Zwart, S.R.; Smith, S.M. Space food for thought: Challenges and considerations for food and nutrition on exploration missions. J. Nutr. 2020, 150, 2242–2244. [Google Scholar] [CrossRef]
- Byard, R.W. Death in the Arctic—The tragic fate of members of the Franklin expedition (1845). Forensic Sci. Med. Pathol. 2020. [Google Scholar] [CrossRef]
- Sempsrott, D. NASA Testing Method to Grow Bigger Plants in Space 2019. Available online: https://www.nasa.gov/feature/nasa-testing-method-to-grow-bigger-plants-in-space (accessed on 22 September 2020).
- Hofer, S.; Honegger, F.; Züger, G. A method to Benchmark Swiss Hospital catering. Int. J. Facil. Manag. 2013, 4. [Google Scholar]
- Edwards, J.S.A.; Hartwell, H. Hospital food service: A comparative analysis of systems and introducing the ’Steamplicity’ concept. J. Hum. Nutr. Diet. 2006, 19, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerreiro, T.M.; De Oliveira, D.N.; Melo, C.F.O.R.; Lima, E.D.O.; Catharino, R.R. Migration from plastic packaging into meat. Food Res. Int. 2018, 109, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The Hazard Analysis and Critical Control Point (HACCP) System. Food Quality and Safety Systems—A Training Manual on Food Hygiene and the Hazard Analysis and Critical Control Point (HACCP) System; FAO Information Division, Publishing Management Group: Rome, Italy, 1998. [Google Scholar]
- Salgueiro, L.; Martins, A.P.; Correia, H. Raw materials: The importance of quality and safety. A review. Flavour Fragr. J. 2010, 25, 253–271. [Google Scholar] [CrossRef]
- Maunder, K.; Lazarus, C.; Walton, K.; Williams, P.; Ferguson, M.; Beck, E. Energy and protein intake increases with an electronic bedside spoken meal ordering system compared to a paper menu in hospital patients. Clin. Nutr. ESPEN 2015, 10, e134–e139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCray, S.; Maunder, K.; Norris, R.; Moir, J.; MacKenzie-Shalders, K. Bedside Menu Ordering System increases energy and protein intake while decreasing plate waste and food costs in hospital patients. Clin. Nutr. ESPEN 2018, 26, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Rask, D.M.G.; Puntel, M.R.; Patzkowski, J.C.; Patzkowski, M.S. Multivitamin use in enhanced recovery after surgery protocols: A cost analysis. Mil. Med. 2020. [Google Scholar] [CrossRef]
- Fatima, M.; Brennan-Olsen, S.L.; Duque, G. Therapeutic approaches to osteosarcopenia: Insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, M.; Lombardi, G.; Sansoni, V.; Perego, S.; Colonna, V.D.G.; Stella, D.; Colombo, C.; Bonadies, M.; De Blasio, G.; Banfi, G.; et al. Vitamin D, cardio-inflammation, and endothelial dysfunction in older adults after orthopedic surgery: Results from an open-label trial to ameliorate cardiac function. Nutr. Clin. Métabolisme 2020, 34, 313–318. [Google Scholar] [CrossRef]
- Purcell, S.; Thornberry, R.; Elliott, S.A.; Panton, L.; Ormsbee, M.J.; Vieira, E.R.; Kim, J.-S.; Prado, C.M. Body composition, strength, and dietary intake of patients with hip or knee osteoarthritis. Can. J. Diet. Pr. Res. 2016, 77, 98–102. [Google Scholar] [CrossRef]
- Morales-Ivorra, I.; Baures, M.R.; Román-Viñas, B.; Serra-Majem, L. Osteoarthritis and the Mediterranean diet: A systematic review. Nutrients 2018, 10, 1030. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; You, Y.; Jun, S. Supercooling preservation technology in food and biological samples: A review focused on electric and magnetic field applications. Food Sci. Biotechnol. 2020, 29, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Lanciotti, R. Applications of high and ultra high pressure homogenization for food safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef] [Green Version]
- Joussain, P.; Thevenet, M.; Rouby, C.; Bensafi, M. Effect of aging on hedonic appreciation of pleasant and unpleasant odors. PLoS ONE 2013, 8, e61376. [Google Scholar] [CrossRef] [Green Version]
- Iannilli, E.; Broy, F.; Kunz, S.; Hummel, T. Age-related changes of gustatory function depend on alteration of neuronal circuits. J. Neurosci. Res. 2017, 95, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Mathey, M.-F.A.; Siebelink, E.; De Graaf, C.; Van Staveren, W.A. Flavor enhancement of food improves dietary intake and nutritional status of elderly nursing home residents. J. Gerontol. Ser. A 2001, 56, M200–M205. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Pelullo, C.P.; Attena, F. Patient evaluation of food waste in three hospitals in southern Italy. Int. J. Environ. Res. Public Health 2019, 16, 4330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.J.; Bauer, J.; Capra, S.; Pulle, R.C. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients—Results of a pragmatic intervention. Clin. Nutr. 2014, 33, 1101–1107. [Google Scholar] [CrossRef]
- Perchonok, M.; Bourland, C. NASA food systems: Past, present, and future. Nutrition 2002, 18, 913–920. [Google Scholar] [CrossRef]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Lippi, G.; Dina, C.Z.; Galentino, R.; Porta, M. Dietary neurotransmitters: A narrative review on current knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [Green Version]
- Zabel, P.; Bamsey, M.; Schubert, D.; Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, F.; De La Torre, A.; Afolayan, E.M.; Olalekan-Ajayi, B.M.; Dhital, B.; Mora-Almanza, J.G.; Potrivitu, G.; Creech, J.; Rivolta, A. Space food and nutrition in a long term manned mission. Adv. Astronaut. Sci. Technol. 2018, 1, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Carillo, P.; Morrone, B.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Challenges for a sustainable food production system on board of the international space station: A technical review. Agronomy 2020, 10, 687. [Google Scholar] [CrossRef]
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space radiation biology for “Living in Space”. BioMed Res. Int. 2020, 2020, 4703286. [Google Scholar] [CrossRef] [Green Version]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D. Losses, inefficiencies and waste in the global food system. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Yu, X.; Liu, H. Insect food for astronauts: Gas exchange in silkworms fed on mulberry and lettuce and the nutritional value of these insects for human consumption during deep space flights. Bull. Entomol. Res. 2011, 101, 613–622. [Google Scholar] [CrossRef]
- NASA. Kennedy Space Center 2020. Available online: https://www.nasa.gov/centers/kennedy/exploration/researchtech/index.html (accessed on 22 September 2020).
- Space Foodsphere—Co-creating the future of Earth, Food and Human Life from Space. Available online: https://spacefoodsphere.jp/en/ (accessed on 22 September 2020).
- Khodadad, C.L.M.; Hummerick, M.E.; Spencer, L.E.; Dixit, A.R.; Richards, J.T.; Romeyn, M.W.; Smith, T.M.; Wheeler, R.M.; Massa, G. Microbiological and nutritional analysis of lettuce crops grown on the international space station. Front. Plant Sci. 2020, 11, 199. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Xu, K. Effects of simulated microgravity on the performance of the duckweeds Lemna aequinoctialis and Wolffia globosa. Aquat. Bot. 2017, 137, 65–71. [Google Scholar] [CrossRef]
- Sexton, A.E.; Garnett, T.; Lorimer, J. Framing the future of food: The contested promises of alternative proteins. Environ. Plan. E Nat. Space 2019, 2, 47–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.; Perchonok, M.; Douglas, G.L. Initial assessment of the nutritional quality of the space food system over three years of ambient storage. NPJ Microgravity 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; Lopez-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef]
- Pandey, M.K.; Gupta, S.C.; Karelia, D.; Gilhooley, P.J.; Shakibaei, M.; Aggarwal, B.B. Dietary nutraceuticals as backbone for bone health. Biotechnol. Adv. 2018, 36, 1633–1648. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Heer, M.A.; Shackelford, L.C.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef]
- Hackney, K.J.; English, K.L. Protein and essential amino acids to protect musculoskeletal health during spaceflight: Evidence of a paradox? Life 2014, 4, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, G.; Dong, D.; Shang, P. Effects of iron overload and oxidative damage on the musculoskeletal system in the space environment: Data from spaceflights and ground-based simulation models. Int. J. Mol. Sci. 2018, 19, 2608. [Google Scholar] [CrossRef] [Green Version]
- Stein, T. Nutrition in the space station era. Nutr. Res. Rev. 2001, 14, 87–118. [Google Scholar] [CrossRef]
- Briguglio, M.; Bona, A.R.; Porta, M.; Dell’osso, B.; Pregliasco, F.E.; Banfi, G. Disentangling the hypothesis of host dysosmia and SARS-CoV-2: The bait symptom that hides neglected neurophysiological routes. Front. Physiol. 2020, 11, 671. [Google Scholar] [CrossRef]
- Shelhamer, M.; Bloomberg, J.; Leblanc, A.; Prisk, G.K.; Sibonga, J.; Smith, S.M.; Zwart, S.R.; Norsk, P. Selected discoveries from human research in space that are relevant to human health on Earth. npj Microgravity 2020, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Boylan, S.M.; Thow, A.-M.; Tyedmers, E.K.; Malik, A.; Salem, J.; Alders, R.; Raubenheimer, D.; Lenzen, M. Using input-output analysis to measure healthy, sustainable food systems. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Cucurachi, S.; Scherer, L.; Guinée, J.; Tukker, A. Life cycle assessment of food systems. One Earth 2019, 1, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, M.; Giorgino, R.; Dell’Osso, B.; Cesari, M.; Porta, M.; Lattanzio, F.; Banfi, G.; Peretti, G.M. Consequences for the elderly after COVID-19 Isolation: FEaR (Frail Elderly amid Restrictions). Front. Psychol. 2020, 11, 565052. [Google Scholar] [CrossRef] [PubMed]
- Fagundes Belchior, G.; Kirk, B.; Pereira da Silva, E.A.; Duque, G. Osteosarcopenia: Beyond age-related muscle and bone loss. Eur. Geriatr. Med. 2020, 11, 715–724. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA 2011, 306, 1782–1793. [Google Scholar] [CrossRef]
- Friedman, S.M.; Mendelson, D.A.; Bingham, K.W.; McCann, R.M. Hazards of hospitalization: Residence prior to admission predicts outcomes. Gerontologist 2008, 48, 537–541. [Google Scholar] [CrossRef]
- Ryback, R.S.; Trimble, R.W.; Lewis, O.F.; Jennings, C.L. Psychobiologic effects of prolonged weightlessness (bed rest) in young healthy volunteers. Aerosp. Med. 1971, 42, 408–415. [Google Scholar]
- Page, N. Weightlessness: A matter of gravity. N. Engl. J. Med. 1977, 297, 32–37. [Google Scholar]
- Corcoran, P.J. Use it or lose it—The hazards of bed rest and inactivity. West. J. Med. 1991, 154, 536–538. [Google Scholar]
- Creditor, M.C. Hazards of hospitalization of the elderly. Ann. Intern. Med. 1993, 118, 219–223. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.D.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Ackermann, G.; Khailova, L.; Baird, C.; Heyland, D.; Kozar, R.; Lemieux, M.; Derenski, K.; King, J.; Vis-Kampen, C.; et al. Extreme dysbiosis of the microbiome in critical illness. mSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, K.; Wikström, L.; Fridlund, R.E.B.; Årestedt, K.; Broström, A. Association of pain ratings with the prediction of early physical recovery after general and orthopaedic surgery—A quantitative study with repeated measures. J. Adv. Nurs. 2017, 73, 2664–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, K.; Årestedt, K.; Broström, A.; Wikström, L. Nausea intensity as a reflector of early physical recovery after surgery. J. Adv. Nurs. 2019, 75, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Falzone, E.; Hoffmann, C.; Keita, H. Postoperative analgesia in elderly patients. Drugs Aging 2013, 30, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, B.L. Loneliness. Easing the pain of the hospitalized elderly. J. Gerontol. Nurs. 1989, 15, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Dunsky, A. The effect of balance and coordination exercises on quality of life in older adults: A mini-review. Front. Aging Neurosci. 2019, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, E.R.; Frett, T.; Haggard, P.; Longo, M.R. A gravitational contribution to perceived body weight. Sci. Rep. 2019, 9, 11448. [Google Scholar] [CrossRef]
- Bizzarri, M.; Monici, M.; Van Loon, J.J.W.A. How microgravity affects the biology of living systems. BioMed Res. Int. 2015, 2015, 863075. [Google Scholar] [CrossRef]
- Stein, T.P. Weight, muscle and bone loss during space flight: Another perspective. Graefe Arch. Clin. Exp. Ophthalmol. 2013, 113, 2171–2181. [Google Scholar] [CrossRef]
- Fitts, R.H.; Trappe, S.W.; Costill, D.L.; Gallagher, P.M.; Creer, A.C.; Colloton, P.A.; Peters, J.R.; Romatowski, J.G.; Bain, J.L.; Riley, D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010, 588, 3567–3592. [Google Scholar] [CrossRef] [PubMed]
- English, K.L.; Bloomberg, J.J.; Mulavara, A.P.; Ploutz-Snyder, L.L. Exercise countermeasures to neuromuscular deconditioning in spaceflight. Compr. Physiol. 2019, 10, 171–196. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Caiani, E.G.; Landreani, F.; Martínez, J.P.; Pueyo, E. Long-term microgravity exposure increases ecg repolarization instability manifested by low-frequency oscillations of T-wave vector. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.T.; Stainforth, R.; Miller, J.; Cahill, T.; Da Silveira, W.A.; Rathi, K.S.; Hardiman, G.; Taylor, D.M.; Costes, S.V.; Chauhan, V.; et al. NASA GeneLab platform utilized for biological response to space radiation in animal models. Cancers 2020, 12, 381. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.-R. The Impact of oxidative stress on the bone system in response to the space special environment. Int. J. Mol. Sci. 2017, 18, 2132. [Google Scholar] [CrossRef] [Green Version]
- Pasiakos, S.M. Nutritional requirements for sustaining health and performance during exposure to extreme environments. Annu. Rev. Nutr. 2020, 40, 221–245. [Google Scholar] [CrossRef]
- Lane, H.W.; Smith, S.M.; Rice, B.L.; Bourland, C.T. Nutrition in space: Lessons from the past applied to the future. Am. J. Clin. Nutr. 1994, 60, 801S–805S. [Google Scholar] [CrossRef] [Green Version]
- Crucian, B.E.; Chouker, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune system dysregulation during spaceflight: Potential countermeasures for deep space exploration missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of aging: Risk factors, consequences, and potential treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-hydroxy-β-methylbutyrate (HMB) on sarcopenia and functional frailty in older persons: A systematic review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment—Facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briguglio, M.; Vitale, J.A.; Galentino, R.; Banfi, G.; Dina, C.Z.; Bona, A.; Panzica, G.; Porta, M.; Dell’Osso, B.; Glick, I.D. Healthy eating, physical activity, and sleep hygiene (HEPAS) as the winning triad for sustaining physical and mental health in patients at risk for or with neuropsychiatric disorders: Considerations for clinical practice. Neuropsychiatr. Dis. Treat. 2020, 16, 55–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, G.C.; Moon, K.H. Effect of osteosarcopenia on postoperative functional outcomes and subsequent fracture in elderly hip fracture patients. Geriatr. Orthop. Surg. Rehabil. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.O.; Sreekanta, A.; Walkeden, S.; Penhale, B.; Hanson, S. Interventions for reducing hospital-associated deconditioning: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 90, 104176. [Google Scholar] [CrossRef] [PubMed]
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry immersion as a ground-based model of microgravity physiological effects. Front. Physiol. 2019, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Paintin, J.; Cooper, C.; Dennison, E. Osteosarcopenia. Br. J. Hosp. Med. 2018, 79, 253–258. [Google Scholar] [CrossRef]
- Obrist, M.; Tu, Y.; Yao, L.; Velasco, C. Space food experiences: Designing passenger’s eating experiences for future space travel scenarios. Front. Comput. Sci. 2019, 1. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [CrossRef]
- Vitale, J.A.; Briguglio, M.; Galentino, R.; Dell’Osso, B.; Malgaroli, A.; Lippi, G.; Porta, M. Exploring circannual rhythms and chronotype effect in patients with Obsessive-Compulsive Tic Disorder (OCTD): A pilot study. J. Affect. Disord. 2020, 262, 286–292. [Google Scholar] [CrossRef]
- Amidon, G.L.; Debrincat, G.A.; Najib, N. Effects of gravity on gastric emptying, intestinal transit, and drug absorption. J. Clin. Pharmacol. 1991, 31, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Millet, C.; Custaud, M.-A.; Maillet, A.; Allevard, A.-M.; Duvareille, M.; Gauquelin-Koch, G.; Gharib, C.; Fortrat, J.-O. Endocrine responses to 7 days of head-down bed rest and orthostatic tests in men and women. Clin. Physiol. 2001, 21, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Stork, C.A.; Sayoc-Becerra, A.; Marchelletta, R.R.; Prisk, G.K.; McCole, D.F. A simulated microgravity environment causes a sustained defect in epithelial barrier function. Sci. Rep. 2019, 9, 17531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergouignan, A.; Stein, T.P.; Habold, C.; Coxam, V.; Gorman, D.O.; Blanc, S. Towards human exploration of space: The THESEUS review series on nutrition and metabolism research priorities. npj Microgravity 2016, 2, 16029. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.E.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Serpe, L.; Canaparo, R.; Dell’Osso, B.; Galentino, R.; De Michele, S.; Dina, C.Z.; Porta, M.; et al. Food bioactive compounds and their interference in drug pharmacokinetic/pharmacodynamic profiles. Pharmaceutics 2018, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Barker, P.D. Reduced G tolerance associated with supplement use. Aviat. Space Environ. Med. 2011, 82, 140–143. [Google Scholar] [CrossRef]
- Sather, T.E.; Woolsey, C.L.; Cromartie, F.; Evans, M.W. The safety and effectiveness of supplement use in aviation. Sport J. 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briguglio, M. Nutritional Orthopedics and Space Nutrition as Two Sides of the Same Coin: A Scoping Review. Nutrients 2021, 13, 483. https://doi.org/10.3390/nu13020483
Briguglio M. Nutritional Orthopedics and Space Nutrition as Two Sides of the Same Coin: A Scoping Review. Nutrients. 2021; 13(2):483. https://doi.org/10.3390/nu13020483
Chicago/Turabian StyleBriguglio, Matteo. 2021. "Nutritional Orthopedics and Space Nutrition as Two Sides of the Same Coin: A Scoping Review" Nutrients 13, no. 2: 483. https://doi.org/10.3390/nu13020483
APA StyleBriguglio, M. (2021). Nutritional Orthopedics and Space Nutrition as Two Sides of the Same Coin: A Scoping Review. Nutrients, 13(2), 483. https://doi.org/10.3390/nu13020483