Evidence for the Contribution of Gut Microbiota to Age-Related Anabolic Resistance
Abstract
:1. Introduction
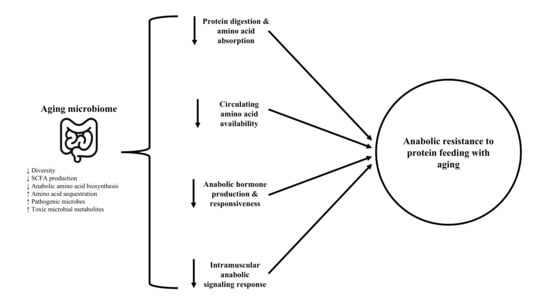
2. The Aging Gut Microbiome & Anabolic Sensitivity
2.1. Overview of the Aging Microbiome, Anabolic Resistance, & Sarcopenia
2.2. Protein Digestion & Amino Acid Absorption
2.3. Circulating Amino Acid Availability
2.4. Anabolic Hormone Responses
2.5. Intramuscular Signaling
3. Lifestyle Factors Contributing to Age-Related Microbiome Changes
3.1. Diet
3.2. Exercise
3.3. Sleep
3.4. Polypharmacy
4. Perspectives and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Colby, S.; Ortman, J. The Baby Boom Cohort in the Unites States: 2012 to 2060; Administration, E.A.S., Ed.; U.S. Census Bureau: Washington, DC, USA, 2014. Available online: https://www.census.gov/prod/2014pubs/p25-1141.pdf (accessed on 22 August 2020).
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawthon, P.M.; Manini, T.; Ms, S.M.P.; Newman, A.; Travison, T.; Kiel, D.P.; Santanasto, A.J.; Ensrud, K.E.; Xue, Q.; Shardell, M.; et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J. Am. Geriatr. Soc. 2020, 68, 1429–1437. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorrens, J.; Rennie, M.J. Effects of ageing and human whole body and muscle protein turnover. Scand. J. Med. Sci. Sports 2003, 13, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Gorissen, S.H.; Van Loon, L.J. Anabolic Resistance of Muscle Protein Synthesis with Aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Rennie, M.J. Anabolic resistance: The effects of aging, sexual dimorphism, and immobilization on human muscle protein turnoverThis paper is one of a selection of papers published in this Special Issue, entitled 14th International Biochemistry of Exercise Conference—Muscles as Molecular and Metabolic Machines, and has undergone the Journal’s usual peer review process. Appl. Physiol. Nutr. Metab. 2009, 34, 377–381. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2004, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Sheffield-Moore, M.; Rasmussen, B.B.; Wolfe, R.R. Basal Muscle Amino Acid Kinetics and Protein Synthesis in Healthy Young and Older Men. JAMA 2001, 286, 1206–1212. [Google Scholar] [CrossRef]
- Dardevet, D.; Sornet, C.; Balage, M.; Grizard, J. Stimulation of In Vitro Rat Muscle Protein Synthesis by Leucine Decreases with Age. J. Nutr. 2000, 130, 2630–2635. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Casperson, S.L.; Dillon, E.L.; Keske, M.A.; Paddon-Jones, D.; Sanford, A.P.; Hickner, R.C.; Grady, J.J.; Sheffield-Moore, M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010, 24, 4117–4127. [Google Scholar] [CrossRef]
- Haran, P.H.; Rivas, D.A.; Fielding, R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J. Cachex-Sarcopenia Muscle 2012, 3, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, Y.; Nourmahnad, A.; Sinha, I. Optimizing Skeletal Muscle Anabolic Response to Resistance Training in Aging. Front. Physiol. 2020, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- De Sire, R.; Rizzatti, G.; Ingravalle, F.; Pizzoferrato, M.; Petito, V.; Lopetuso, L.; Graziani, C.; De Sire, A.; Mentella, M.C.; Mele, M.C.; et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol. Dietol. 2018, 64, 351–362. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Steves, C.J. Dietary Protein and Muscle in Aging People: The Potential Role of the Gut Microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [Green Version]
- Lustgarten, M.S. The Role of the Gut Microbiome on Skeletal Muscle Mass and Physical Function: 2019 Update. Front. Physiol. 2019, 10, 1435. [Google Scholar] [CrossRef] [Green Version]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Enoki, Y.; Watanabe, H.; Arake, R.; Sugimoto, R.; Imafuku, T.; Tominaga, Y.; Ishima, Y.; Kotani, S.; Nakajima, M.; Tanaka, M.; et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci. Rep. 2016, 6, 32084. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.D.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Heintz, C.; Mair, W. You Are What You Host: Microbiome Modulation of the Aging Process. Cell 2014, 156, 408–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, P.W.; Jeffery, I.B. Microbiome–health interactions in older people. Cell. Mol. Life Sci. 2018, 75, 119–128. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willing, B.P.; Van Kessel, A.G. Intestinal microbiota differentially affect brush border enzyme activity and gene expression in the neonatal gnotobiotic pig. J. Anim. Physiol. Anim. Nutr. 2009, 93, 586–595. [Google Scholar] [CrossRef]
- Kozakova, H.; Kolinska, J.; Lojda, Z.; Rehakova, Z.; Sinkora, J.; Zákostelecká, M.; Šplíchal, I.; Tlaskalova-Hogenova, H. Effect of bacterial monoassociation on brush-border enzyme activities in ex-germ-free piglets: Comparison of commensal and pathogenic Escherichia coli strains. Microbes Infect. 2006, 8, 2629–2639. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Giebultowicz, J.; Wroczynski, P.; Paczek, L. Age-dependent increase in serum levels of indoxyl sulphate and p-cresol sulphate is not related to their precursors: Tryptophan and tyrosine. Geriatr. Gerontol. Int. 2016, 17, 1022–1026. [Google Scholar] [CrossRef]
- Dallas, D.C.; Sanctuary, M.R.; Qu, Y.; Khajavi, S.H.; Van Zandt, A.E.; Dyandra, M.; Frese, S.A.; Barile, D.; German, J.B. Personalizing protein nourishment. Crit. Rev. Food Sci. Nutr. 2017, 57, 3313–3331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Rampelli, S.; Candela, M.; Turroni, S.; Biagi, E.; Collino, S.; Franceschi, C.; O’Toole, P.W.; Brigidi, P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging 2013, 5, 902–912. [Google Scholar] [CrossRef] [Green Version]
- Torrallardona, D.; Harris, C.I.; Coates, M.E.; Fuller, M.F. Microbial amino acid synthesis and utilization in rats: Incorporation of15N from15NH4Cl into lysine in the tissues of germ-free and conventional rats. Br. J. Nutr. 1996, 76, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metges, C.C.; El-Khoury, A.E.; Henneman, L.; Petzke, K.J.; Grant, I.; Bedri, S.; Pereira, P.P.; Ajami, A.M.; Fuller, M.F.; Young, V.R. Availability of intestinal microbial lysine for whole body lysine homeostasis in human subjects. Am. J. Physiol. Metab. 1999, 277, E597–E607. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Sitaraman, R. Aging and the human gut microbiota—from correlation to causality. Front. Microbiol. 2015, 5, 764. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Raj, T.; Dileep, U.; Vaz, M.; Fuller, M.F.; Kurpad, A.V. Intestinal Microbial Contribution to Metabolic Leucine Input in Adult Men. J. Nutr. 2008, 138, 2217–2221. [Google Scholar] [CrossRef] [Green Version]
- Neis, E.P.J.G.; DeJong, C.H.C.; Rensen, S.S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpi, E.; Mittendorfer, B.; Wolf, S.E.; Wolfe, R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol. Content 1999, 277, E513–E520. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.; Burrin, D.G. Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers1,2. J. Anim. Sci. 2006, 84, E60–E72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullman, E.L.; Campbell, W.W.; Krishnan, R.K.; Yarasheski, K.E.; Evans, W.J.; Kirwan, J.P. Age Attenuates Leucine Oxidation after Eccentric Exercise. Int. J. Sports Med. 2013, 34, 695–699. [Google Scholar] [CrossRef] [Green Version]
- Kurpad, A.V.; Jahoor, F.; Borgonha, S.; Poulo, S.; Rekha, S.; Fjeld, C.R.; Reeds, P.J. A minimally invasive tracer protocol is effective for assessing the response of leucine kinetics and oxidation to vaccination in chronically energy-deficient adult males and children. J. Nutr. 1999, 129, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Battson, M.L.; Lee, D.M.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am. J. Physiol. Metab. 2018, 314, E468–E477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Richey, J.J.; Zigler, M.C.; Cuevas, L.M.; Gonzalez, A.; Vázquez-Baeza, Y.; Battson, M.L.; Smithson, A.T.; Gilley, A.D.; et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 2019, 597, 2361–2378. [Google Scholar] [CrossRef] [Green Version]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; Vandongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef] [Green Version]
- Tumur, Z.; Niwa, T. Indoxyl Sulfate Inhibits Nitric Oxide Production and Cell Viability by Inducing Oxidative Stress in Vascular Endothelial Cells. Am. J. Nephrol. 2009, 29, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals through TOR-Dependent Nutrient Sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, M.E.; Balmand, S.; Hudcovic, T.; Heddi, A.; et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Veter. Res. 2019, 15, 315. [Google Scholar] [CrossRef]
- Woodmansey, E. Intestinal bacteria and ageing. J. Appl. Microbiol. 2007, 102, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.; Sharp, R.; Macfarlane, G.T. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001, 48, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, A.A.; Sovran, B.; Hugenholtz, F.; Meijer, B.; Hoogerland, J.A.; Mihailova, V.; Van Der Ploeg, C.; Belzer, C.; Boekschoten, M.V.; Hoeijmakers, J.H.J.; et al. Supplementation with Lactobacillus plantarum WCFS1 Prevents Decline of Mucus Barrier in Colon of Accelerated Aging Ercc1−/Δ7 Mice. Front. Immunol. 2016, 7, 408. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Friedrich, N.; Thuesen, B.; Jørgensen, T.; Juul, A.; Spielhagen, C.; Wallaschofksi, H.; Linneberg, A. The Association Between IGF-I and Insulin Resistance: A general population study in Danish adults. Diabetes Care 2012, 35, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimball, S.R. Interaction between the AMP-Activated Protein Kinase and mTOR Signaling Pathways. Med. Sci. Sports Exerc. 2006, 38, 1958–1964. [Google Scholar] [CrossRef] [Green Version]
- Nobukuni, T.; Joaquin, M.; Roccio, M.; Dann, S.G.; Kim, S.Y.; Gulati, P.; Byfield, M.P.; Backer, J.M.; Natt, F.; Bos, J.L.; et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 14238–14243. [Google Scholar] [CrossRef] [Green Version]
- Grosicki, G.J.; Barrett, B.B.; Englund, D.A.; Liu, C.; Travison, T.G.; Cederholm, T.; Koochek, A.; Von Berens, Å.; Gustafsson, T.; Benard, T.; et al. Circulating Interleukin-6 Is Associated with Skeletal Muscle Strength, Quality, and Functional Adaptation with Exercise Training in Mobility-Limited Older Adults. J. Frailty Aging 2020, 9, 57–63. [Google Scholar]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.A.; Lang, C.H. mTor Signaling in Skeletal Muscle During Sepsis and Inflammation: Where Does It All Go Wrong? Physiology 2011, 26, 83–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiano, S.; Muscariello, E.; La Rosa, G.; Di Maro, M.; Mondola, P.; Santillo, M. Dual Role of Reactive Oxygen Species in Muscle Function: Can Antioxidant Dietary Supplements Counteract Age-Related Sarcopenia? Int. J. Mol. Sci. 2019, 20, 3815. [Google Scholar] [CrossRef] [Green Version]
- Mercante, J.W.; Neish, A.S. Reactive Oxygen Production Induced by the Gut Microbiota: Pharmacotherapeutic Implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Walrand, S.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.M.; Van Loon, L.J.C. Dietary Protein Digestion and Absorption Rates and the Subsequent Postprandial Muscle Protein Synthetic Response Do Not Differ between Young and Elderly Men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Gibson, J.A.; Sladen, G.E.; Dawson, A.M. Protein absorption and ammonia production: The effects of dietary protein and removal of the colon. Br. J. Nutr. 1976, 35, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, N.R.; Fereday, A.; Cox, M.; Halliday, D.; Pacy, P.J.; Millward, D.J. Influences of dietary energy and protein on leucine kinetics during feeding in healthy adults. Am. J. Physiol. Metab. 1996, 270, E282–E291. [Google Scholar] [CrossRef]
- Dangin, M.; Guillet, C.; Garcia-Rodenas, C.; Gachon, P.; Bouteloup-Demange, C.; Reiffers-Magnani, K.; Fauquant, J.; Ballèvre, O.; Beaufrère, B. The Rate of Protein Digestion affects Protein Gain Differently during Aging in Humans. J. Physiol. 2003, 549, 635–644. [Google Scholar] [CrossRef]
- Milan, A.M.; D’Souza, R.F.; Pundir, S.; Pileggi, C.A.; Barnett, M.P.G.; Markworth, J.F.; Cameronsmith, D.; Mitchell, C.J. Older adults have delayed amino acid absorption after a high protein mixed breakfast meal. J. Nutr. Health Aging 2015, 19, 839–845. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van De Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2018, 20, 145–154. [Google Scholar] [CrossRef]
- Raul, F.; Gosse, F.; Doffoel, M.; Darmenton, P.; Wessely, J.Y. Age related increase of brush border enzyme activities along the small intestine. Gut 1988, 29, 1557–1563. [Google Scholar] [CrossRef] [Green Version]
- Feldman, M.; Cryer, B.; McArthur, K.E.; Huet, B.A.; Lee, E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: A prospective study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.B.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Debebe, T.; Biagi, E.; Soverini, M.; Holtze, S.; Hildebrandt, T.B.; Birkemeyer, C.; Wyohannis, D.; Lemma, A.; Brigidi, P.; Savkovic, V.; et al. Unraveling the gut microbiome of the long-lived naked mole-rat. Sci. Rep. 2017, 7, 9590. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Zeng, T.; Zinellu, A.; Rubino, S.; Kelvin, D.J.; Carru, C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems 2019, 4, e00325-19. [Google Scholar] [CrossRef] [Green Version]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Metges, C.C. Contribution of Microbial Amino Acids to Amino Acid Homeostasis of the Host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Huang, G.; Cai, D.; Li, D.; Liang, X.; Yu, T.; Shen, P.; Su, H.; Liu, J.; Gu, H.; et al. Qualitative and Semiquantitative Analysis of Fecal Bifidobacterium Species in Centenarians Living in Bama, Guangxi, China. Curr. Microbiol. 2015, 71, 143–149. [Google Scholar] [CrossRef]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Li, R.; Lin, H.; Fu, C.; Wang, X.; Zhang, Y.; Su, M.; Huang, P.; Qian, J.; Jiang, F.; et al. Enriched taxa were found among the gut microbiota of centenarians in East China. PLoS ONE 2019, 14, e0222763. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-S.; Choi, C.W.; Shin, H.; Jin, S.-P.; Bae, J.-S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups. J. Microbiol. Biotechnol. 2019, 29, 429–440. [Google Scholar] [CrossRef]
- Matteuzzi, D.; Crociani, F.; Emaldi, O. Amino acids produced by bifidobacteria and some Clostridia. Ann. Microbiol. 1978, 129, 175–181. [Google Scholar]
- Sauer, F.D.; Erfle, J.D.; Mahadevan, S. Amino acid biosynthesis in mixed rumen cultures. Biochem. J. 1975, 150, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Tesseraud, S.; Peresson, R.; Lopes, J.; Chagneau, A. Dietary lysine deficiency greatly affects muscle and liver protein turnover in growing chickens. Br. J. Nutr. 1996, 75, 853–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Murabito, J.M.; Rhee, E.P.; Ho, J.E.; Jacques, P.F.; Ghorbani, A.; Magnusson, M.; Souza, A.L.; et al. Distinct metabolomic signatures are associated with longevity in humans. Nat. Commun. 2015, 6, 6791. [Google Scholar] [CrossRef]
- Van Tongeren, S.P.; Slaets, J.P.J.; Harmsen, H.J.M.; Welling, G.W.; Viterbo, A.; Harel, M.; Horwitz, B.A.; Chet, I.; Mukherjee, P.K. Fecal Microbiota Composition and Frailty. Appl. Environ. Microbiol. 2005, 71, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Eliasson, J.; Karlsson, H.K.R.; Köhnke, R. Branched-Chain Amino Acids Activate Key Enzymes in Protein Synthesis after Physical Exercise. J. Nutr. 2006, 136, 269S–273S. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.; Cynober, L.; Moinard, C.; Blanc, M.C.; Neveux, N.; De Bandt, J.P.; Aussel, C. Splanchnic sequestration of amino acids in aged rats: In vivo and ex vivo experiments using a model of isolated perfused liver. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R748–R755. [Google Scholar] [CrossRef] [Green Version]
- Boirie, Y.; Gachon, P.; Beaufrère, B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J. Clin. Nutr. 1997, 65, 489–495. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ouwehand, A.C.; Isolauri, E.; Hosoda, M.; Benno, Y.; Salminen, S. Differences in Composition and Mucosal Adhesion of Bifidobacteria Isolated from Healthy Adults and Healthy Seniors. Curr. Microbiol. 2001, 43, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Hoffman, J.R.; Stout, J.R.; Fukuda, D.H.; Willoughby, D.S. Intramuscular Anabolic Signaling and Endocrine Response Following Resistance Exercise: Implications for Muscle Hypertrophy. Sports Med. 2016, 46, 671–685. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Grant, E.J.; Correia, C.E.; Phillips, S.M. Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 98, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Anderson, J.M.; Armstrong, L.E.; Nindl, B.C.; Volek, J.S.; Judelson, D.A.; Joseph, M.; Vingren, J.L.; Hatfield, D.L.; et al. Effects of Elevated Circulating Hormones on Resistance Exercise-Induced Akt Signaling. Med. Sci. Sports Exerc. 2008, 40, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.D.; Kujbida, G.W.; Moore, D.R.; Atherton, P.; Burd, N.A.; Padzik, J.P.; De Lisio, M.; Tang, J.E.; Parise, G.; Rennie, M.J.; et al. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J. Physiol. 2009, 587, 5239–5247. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. Insulin and muscle protein turnover in humans: Stimulatory, permissive, inhibitory, or all of the above? Am. J. Physiol. Metab. 2008, 295, E731. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia Exacerbates Obesity-Associated Insulin Resistance and Dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef]
- Guillet, C.; Boirie, Y. Insulin resistance: A contributing factor to age-related muscle mass loss? Diabetes Metab. 2005, 31, 5S20–5S26. [Google Scholar] [CrossRef]
- Toogood, A.A. Growth hormone (GH) status and body composition in normal ageing and in elderly adults with GH deficiency. Horm. Res. 2003, 60, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Russell-Aulet, M.; Dimaraki, E.V.; Jaffe, C.A.; DeMott-Friberg, R.; Barkan, A.L. Aging-related growth hormone (GH) decrease is a selective hypothalamic GH-releasing hormone pulse amplitude mediated phenomenon. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2001, 56, M124–M129. [Google Scholar] [CrossRef] [Green Version]
- Cappola, A.R.; Xue, Q.-L.; Fried, L.P. Multiple Hormonal Deficiencies in Anabolic Hormones Are Found in Frail Older Women: The Women’s Health and Aging Studies. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2009, 64, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, J.W.; Roffwarg, H.P.; Boyar, R.M.; Kream, J.; Hellman, L. Age-Related Change in the Twenty-Four Hour Spontaneous Secretion of Growth Hormone. J. Clin. Endocrinol. Metab. 1972, 35, 665–670. [Google Scholar] [CrossRef]
- Waters, D.L.; Yau, C.L.; Montoya, G.D.; Baumgartner, R.N. Serum Sex Hormones, IGF-1, and IGFBP3 Exert a Sexually Dimorphic Effect on Lean Body Mass in Aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M648–M652. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Dillon, E.L. Nutritionally essential amino acids and metabolic signaling in aging. Amino Acids 2013, 45, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, B.B.; Fujita, S.; Wolfe, R.R.; Mittendorfer, B.; Roy, M.; Rowe, V.L.; Volpi, E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006, 20, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, U.; Randin, D.; Vollenweider, P.; Vollenweider, L.; Nicod, P. Nitric oxide release accounts for insulin’s vascular effects in humans. J. Clin. Investig. 1994, 94, 2511–2515. [Google Scholar] [CrossRef]
- Petrie, J.R.; Ueda, S.; Webb, D.J.; Elliott, H.L.; Connell, J.M. Endothelial Nitric Oxide Production and Insulin Sensitivity. Circulation 1996, 93, 1331–1333. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Chong, A.L.; Zhang, W.Z.; Kaye, D.M. Insulin-mediated activation of the L-arginine nitric oxide pathway in man, and its impairment in diabetes. PLoS ONE 2013, 8, e61840. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.B.; Cusco, J.A.; Roddy, M.-A.; Johnstone, M.T.; Creager, M.A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1996, 27, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.G.; Wallis, M.G.; Barrett, E.J.; Vincent, M.A.; Richards, S.M.; Clerk, L.H.; Rattigan, S. Blood flow and muscle metabolism: A focus on insulin action. Am. J. Physiol. Metab. 2003, 284, E241–E258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Grady, J.J.; Volpi, E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am. J. Physiol. Metab. 2006, 291, E745–E754. [Google Scholar] [CrossRef] [Green Version]
- Nygren, J.; Nair, K.S. Differential Regulation of Protein Dynamics in Splanchnic and Skeletal Muscle Beds by Insulin and Amino Acids in Healthy Human Subjects. Diabetes 2003, 52, 1377–1385. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, H.O.; Brechtel, G.; Johnson, A.; Fineberg, N.; Baron, A.D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Investig. 1994, 94, 1172–1179. [Google Scholar] [CrossRef]
- Fransen, F.; Van Beek, A.A.; Borghuis, T.; El Aidy, S.; Hugenholtz, F.; Jongh, C.V.D.G.-D.; Savelkoul, H.F.J.; De Jonge, M.I.; Boekschoten, M.V.; Smidt, H.; et al. Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front. Immunol. 2017, 8, 1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallberg, Z.F.; Taga, M.E. Taking the “Me” out of meat: A new demethylation pathway dismantles a toxin’s precursor. J. Biol. Chem. 2020, 295, 11982–11983. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J. Claims for the anabolic effects of growth hormone: A case of the Emperor’s new clothes? Br. J. Sports Med. 2003, 37, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velloso, C.P. Regulation of muscle mass by growth hormone and IGF-I. Br. J. Pharmacol. 2008, 154, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikani, V.; Ho, K.K.Y. Action of GH on skeletal muscle function: Molecular and metabolic mechanisms. J. Mol. Endocrinol. 2013, 52, R107–R123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Wan, X.; Wang, S.; Xu, J.; Zhuang, L.; Xing, K.; Zhang, M.; Zhu, X.; Wang, L.; Gao, P.; Xi, Q.; et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS ONE 2017, 12, e0173174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, U.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino Acid Supplementation Increases Lean Body Mass, Basal Muscle Protein Synthesis, and Insulin-Like Growth Factor-I Expression in Older Women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [Green Version]
- Crowe, F.L.; Key, T.J.; Allen, N.E.; Appleby, P.N.; Roddam, A.; Overvad, K.; Grønbaek, H.; Tjønneland, A.; Halkjaer, J.; Dossus, L.; et al. The Association between Diet and Serum Concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Wolk, K.; Brismar, K.; Wolk, A. Association of diet with serum insulin-like growth factor I in middle-aged and elderly men. Am. J. Clin. Nutr. 2005, 81, 1163–1167. [Google Scholar] [CrossRef] [Green Version]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.S.; Wei, H.K.; Wang, P.; Yu, H.C.; Zhang, X.M.; Jiang, S.W.; Peng, J. Early intervention with faecal microbiota transplantation: An effective means to improve growth performance and the intestinal development of suckling piglets. Animal 2019, 13, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Goodman, C.A.; Frey, J.W.; Mabrey, D.M.; Jacobs, B.L.; Lincoln, H.C.; You, J.-S.; Hornberger, T.A. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J. Physiol. 2011, 589, 5485–5501. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and Downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.-S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [Green Version]
- Reiling, J.H.; Sabatini, D.M. Stress and mTORture signaling. Oncogene 2006, 25, 6373–6383. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Manning, B.D. The TSC1–TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Arranz, L.; Lord, J.M.; De La Fuente, M. Preserved ex vivo inflammatory status and cytokine responses in naturally long-lived mice. AGE 2010, 32, 451–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Salazar, N.; González, S.; Nogacka, A.M.; Rios-Covián, D.; Arboleya, S.; Gueimonde, M.; Reyes-Gavilán, C.G.D.L. Microbiome: Effects of Ageing and Diet. Curr. Issues Mol. Biol. 2020, 36, 33–62. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; Reyes-Gavilán, C.G.D.L. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Tammela, L.; Korpela, J.; Parhiala, R.; Ahokoski, H.; Mykkänen, H.; Salminen, S.J. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. AGE 2008, 31, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampelli, S.; Candela, M.; Severgnini, M.; Biagi, E.; Turroni, S.; Roselli, M.; Carnevali, P.; Donini, L.; Brigidi, P. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J. Nutr. Health Aging 2012, 17, 166–172. [Google Scholar] [CrossRef]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P.; Turroni, S.; Hrelia, S.; Hrelia, P.; Bereswill, S.; Fischer, A.; et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota – The “RISTOMED project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Prasad, J.; Gill, H.; Stevenson, L.; Gopal, P. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J. Nutr. Health Aging 2007, 11, 26–31. [Google Scholar] [PubMed]
- Carter, S.L.; Rennie, C.D.; Hamilton, S.J.; Tarnopolsky. Changes in skeletal muscle in males and females following endurance training. Can. J. Physiol. Pharm. 2001, 79, 386–392. [Google Scholar] [CrossRef]
- Ekblom, B.; Astrand, P.O.; Saltin, B.; Stenberg, J.; Wallström, B. Effect of training on circulatory response to exercise. J. Appl. Physiol. 1968, 24, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Puntschart, A.; Claassen, H.; Jostarndt, K.; Hoppeler, H.; Billeter, R. mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am. J. Physiol. Physiol. 1995, 269, C619–C625. [Google Scholar] [CrossRef]
- Saltin, B.; Blomqvist, G.; Mitchell, J.H.; Johnson, R.L.; Wildenthal, K.; Chapman, C.B. Response to exercise after bed rest and after training. Circulation 1968, 38, Vii1-78. [Google Scholar]
- Evans, J.M.; Morris, L.S.; Marchesi, J.R. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J. Endocrinol. 2013, 218, R37–R47. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, F. Probiotics in inflammatory bowel disease—therapeutic rationale and role. Adv. Drug Deliv. Rev. 2004, 56, 809–818. [Google Scholar] [CrossRef]
- Thompson, W.G.; Longstreth, G.F.; Drossman, D.A.; Heaton, K.W.; Irvine, E.J.; Muller-Lissner, S.A. Functional bowel disorders and functional abdominal pain. Gut 1999, 45, ii43–ii47. [Google Scholar] [CrossRef]
- Bermon, S.; Petriz, B.; Kajeniene, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol Rev. 2015, 21, 70–79. [Google Scholar]
- Mika, A.; Van Treuren, W.; González, A.; Herrera, J.J.; Knight, R.; Fleshner, M. Exercise Is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile versus Adult Male F344 Rats. PLoS ONE 2015, 10, e0125889. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.P.F.; De Vries, W.R.; Vanberge-Henegouwen, G.P.; Akkermans, L.M.A. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001, 48, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary Running Exercise Alters Microbiota Composition and Increases n-Butyrate Concentration in the Rat Cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, E.V.; Grandy, S.A.; Langille, M.G.I. Moderate Exercise Has Limited but Distinguishable Effects on the Mouse Microbiome. mSystems 2017, 2, e00006-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, W.; Cronin, O.; Garcia-Perez, I.; Whiston, R.; Holmes, E.; Woods, T.; Molloy, C.B.; Molloy, M.G.; Shanahan, F.; Cotter, P.D.; et al. The effects of sustained fitness improvement on the gut microbiome: A longitudinal, repeated measures case-study approach. Transl. Sports Med. 2020. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Durk, R.P.; Bagley, J.R. Rapid gut microbiome changes in a world-class ultramarathon runner. Physiol. Rep. 2019, 7, e14313. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Monda, M.; Messina, G.; Scognamiglio, I.; Lombardi, A.; Martin, G.A.; Sperlongano, P.; Porcelli, M.; Caraglia, M.; Stiuso, P. Short-Term Diet and Moderate Exercise in Young Overweight Men Modulate Cardiocyte and Hepatocarcinoma Survival by Oxidative Stress. Oxidative Med. Cell. Longev. 2014, 2014, 131024. [Google Scholar] [CrossRef]
- Liu, J.; Johnson, R.; Dillon, S.; Kroehl, M.; Frank, D.N.; Tuncil, Y.E.; Zhang, X.; Ir, D.; Robertson, C.E.; Seifert, S.; et al. Among older adults, age-related changes in the stool microbiome differ by HIV-1 serostatus. EBioMedicine 2019, 40, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Häggblom, M.M.; Lightfoot, S.A.; Joseph, L.B.; Kerkhof, L.J. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef] [Green Version]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [Green Version]
- Crowley, K. Sleep and Sleep Disorders in Older Adults. Neuropsychol. Rev. 2011, 21, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in Normal Aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Riemann, B.L.; Flatt, A.A.; Valentino, T.; Lustgarten, M.S. Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: A pilot study. Sleep Med. 2020, 73, 76–81. [Google Scholar] [CrossRef]
- Anderson, J.R.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.D.; Heinberg, L.J.; Peat, C.; Steffen, K.; Manderino, L.M.; Mitchell, J.; Gunstad, J. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017, 38, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [Green Version]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schuermann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Liu, Q.-Q.; Su, H.-Z.; Zhang, H.-P.; Fan, J.-M.; Yang, J.-H.; Hu, A.-K.; Liu, Y.-Q.; Chou, D.; Zeng, Y.-M. Gut microbiota in obstructive sleep apnea–hypopnea syndrome: Disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 2019, 133, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Saner, N.J.; Lee, M.J.; Pitchford, N.W.; Kuang, J.; Roach, G.D.; Garnham, A.; Stokes, T.; Phillips, S.M.; Bishop, D.J.; Bartlett, J.D. The effect of sleep restriction, with or without high-intensity interval exercise, on myofibrillar protein synthesis in healthy young men. J. Physiol. 2020, 598, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Chien, M.-Y.; Wang, L.-Y.; Chen, H.-C. The Relationship of Sleep Duration with Obesity and Sarcopenia in Community-Dwelling Older Adults. Gerontology 2015, 61, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.W.; Kelly, J.P.; Rosenberg, L.; Anderson, T.E.; Mitchell, A.A. Recent Patterns of Medication Use in the Ambulatory Adult Population of the United States. JAMA 2002, 287, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.R.; Ramsdale, E.; Loh, K.P.; Arastu, A.; Xu, H.; Obrecht, S.; Castillo, D.; Sharma, M.; Holmes, H.M.; Nightingale, G.; et al. Associations of Polypharmacy and Inappropriate Medications with Adverse Outcomes in Older Adults with Cancer: A Systematic Review and Meta-Analysis. Oncologist 2019, 25, e94–e108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, E.R.; Cafiero, A.C.; Hanlon, J.T. Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 2007, 5, 345–351. [Google Scholar] [CrossRef]
- Gemikonakli, G.; Mach, J.; Hilmer, S.N. Interactions between the Aging Gut Microbiome and Common Geriatric Giants: Polypharmacy, Frailty, and Dementia. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2020. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef] [Green Version]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [Green Version]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Guinan, J.; Wang, S.; Hazbun, T.R.; Yadav, H.; Thangamani, S. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci. Rep. 2019, 9, 8872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nat. Cell Biol. 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]


| Biological Process | Age-Related Change | Suspected Gut Microbiota Contribution |
|---|---|---|
| Protein Digestion & Amino Acid Absorption |
|
|
|
| |
| Circulating Amino Acid Availability |
|
|
|
| |
| Anabolic Hormone Production & Responsiveness |
|
|
|
| |
| Intramuscular Anabolic Signaling Response |
| |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, M.D.; Cross, B.L.; Grosicki, G.J. Evidence for the Contribution of Gut Microbiota to Age-Related Anabolic Resistance. Nutrients 2021, 13, 706. https://doi.org/10.3390/nu13020706
Watson MD, Cross BL, Grosicki GJ. Evidence for the Contribution of Gut Microbiota to Age-Related Anabolic Resistance. Nutrients. 2021; 13(2):706. https://doi.org/10.3390/nu13020706
Chicago/Turabian StyleWatson, Matthew D., Brett L. Cross, and Gregory J. Grosicki. 2021. "Evidence for the Contribution of Gut Microbiota to Age-Related Anabolic Resistance" Nutrients 13, no. 2: 706. https://doi.org/10.3390/nu13020706
APA StyleWatson, M. D., Cross, B. L., & Grosicki, G. J. (2021). Evidence for the Contribution of Gut Microbiota to Age-Related Anabolic Resistance. Nutrients, 13(2), 706. https://doi.org/10.3390/nu13020706