Saccharomyces cerevisiae Yeast-Based Supplementation as a Galactagogue in Breastfeeding Women? A Review of Evidence from Animal and Human Studies
Abstract
:1. Introduction
2. An Overview of Information of Saccharomyces cerevisiae Yeast-Based Supplement on the Internet
3. Using Saccharomyces Cerevisiae Yeast as a Galactagogue in Ruminants and Non-Ruminants
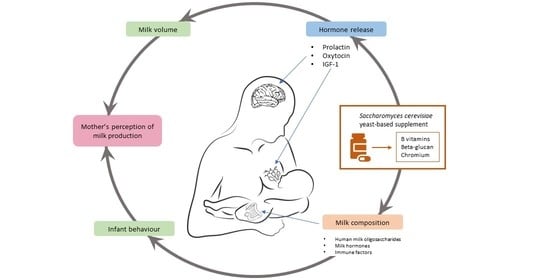
4. Hypotheses of the Mechanism of Saccharomyces cerevisiae Yeast-Based Supplement on Breast Milk Production
5. Safety Considerations of Taking Saccharomyces cerevisiae Yeast-Based Supplement during Lactation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
- A breastfeeding woman (>18 years) consumes 30 g/day SCYS;
- The concentration of nicotinic acid in SCYS is 1 mg/g, as per the highest value in Ahmad and Moat’s study of SCY grown in culture added with 500 µg/mL tryptophan [132].
- A breastfeeding woman (>18 years) consumes 30 g/day SCYS;
- The concentration of folic acid in SCYS is 0.045 mg/g, as per the highest value in Table 2 (75 µg DFE = 45 µg folic acid).
- A breastfeeding woman (>18 years) consumes 30 g/day SCYS;
- The SCYS contains 4.24 ng/g OTA as the highest level reported in Germany study [112];
- The body weight of the woman is 70 kg;
- The consumption of OTA from other source is 2.1 ng/kg body weight/day as the highest OTA dietary exposure for New Zealand women [133].
References
- Odom, E.C.; Li, R.; Scanlon, K.S.; Perrine, C.G.; Grummer-Strawn, L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics 2013, 131, e726–e732. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, R.; Rehman, S.U.; Nawaz, S.; Mohammad, T. Factors causing non-breastfeeding in children under six months of age in district Nowshera, Pakistan. J. Ayub. Med. Coll. Abbottabad JAMC 2010, 21, 93–95. [Google Scholar]
- Camurdan, A.D.; Ilhan, M.N.; Beyazova, U.; Sahin, F.; Vatandas, N.; Eminoglu, S. How to achieve long-term breast-feeding: Factors associated with early discontinuation. Public Health Nutr. 2008, 11, 1173–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaqub, A.; Gul, S. Reasons for failure of exclusive breastfeeding in children less than six months of age. J. Ayub Med. Coll. Abbotabad JAMC 2014, 25, 165–167. [Google Scholar]
- Gatti, L. Maternal perceptions of insufficient milk supply in breastfeeding. J. Nurs. Scholarsh. 2008, 40, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, K.; Dennis, C.-L.; Tatsuoka, H.; Jimba, M. The relationship between breastfeeding self-efficacy and perceived insufficient milk among japanese mothers. J. Obstet. Gynecol. Neonatal Nurs. 2008, 37, 546–555. [Google Scholar] [CrossRef]
- Hurst, N.M. Recognizing and treating delayed or failed lactogenesis II. J. Midwifery Women’s Health 2007, 52, 588–594. [Google Scholar] [CrossRef]
- Whitten, D. A precious opportunity: Supporting women with concerns about their breastmilk supply. Aust. J. Herbal Med. 2013, 25, 1–41. [Google Scholar]
- Institute of Medicine. Nutrition During Lactation; National Academies Press: Washington, DC, USA, 1991; pp. 8–112. [Google Scholar]
- Prentice, A.M.; Roberts, S.B.; Prentice, A.; Paul, A.A.; Watkinson, M.; Watkinson, A.A.; Whitehead, R.G. Dietary supplementation of lactating Gambian women. I. Effect on breast-milk volume and quality. Hum. Nutr. Clin. Nutr. 1983, 37, 53–64. [Google Scholar] [PubMed]
- Dewey, K.G. Effects of maternal caloric restriction and exercise during lactation. J. Nutr. 1998, 128, 386S–389S. [Google Scholar] [CrossRef] [Green Version]
- Bravi, F.; Wiens, F.; DeCarli, A.; Pont, A.D.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and micronutrients of human milk composition: Are they related to maternal diet? A comprehensive systematic review. Breastfeed. Med. 2017, 12, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Safon, C.; Keene, D.; Guevara, W.J.U.; Kiani, S.; Herkert, D.; Muñoz, E.E.; Pérez-Escamilla, R. Determinants of perceived insufficient milk among new mothers in León, Nicaragua. Matern. Child Nutr. 2016, 13, e12369. [Google Scholar] [CrossRef]
- Sacco, L.M.; Caulfield, L.E.; Gittelsohn, J.; Martínez, H. The Conceptualization of perceived insufficient milk among mexican mothers. J. Hum. Lact. 2006, 22, 277–286. [Google Scholar] [CrossRef]
- Peacock-Chambers, E.; Dicks, K.; Sarathy, L.; Brown, A.A.; Boynton-Jarrett, R. Perceived Maternal behavioral control, infant behavior, and milk supply: A qualitative study. J. Dev. Behav. Pediatr. 2017, 38, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Dykes, F.; Williams, C. Falling by the wayside: A phenomenological exploration of perceived breast-milk inadequacy in lactating women. Midwifery 1999, 15, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Rodrigo, A.; Liyanage, N.; Hatahagoda, W.; Hewavitharana, U. Maternal perception of adequacy of mother’s milk among mothers giving birth at a teaching hospital in sri lanka. J. Hum. Lact. 2018, 35, 171–180. [Google Scholar] [CrossRef]
- Forinash, A.B.; Yancey, A.M.; Barnes, K.N.; Myles, T.D. the use of galactogogues in the breastfeeding mother. Ann. Pharmacother. 2012, 46, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Zapantis, A.; Steinberg, J.G.; Schilit, L. Use of herbals as galactagogues. J. Pharm. Pract. 2012, 25, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Westfall, R.E. Galactagogue herbs: A qualitative study and review. Can. J. Midwifery Res. Pract. 2003, 2, 22–27. [Google Scholar]
- Steyn, N.; Zunza, M.; Decloedt, E.H. A cross-sectional descriptive study of breastfeeding behaviour and galactogogue use among private-sector patients in Cape Town, South Africa. S. Afr. J. Obstet. Gynaecol. 2017, 23, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, H. A lactogenic herbal. In Mother Food: Lactogenic Food and Herbs for Milk Production and for a Mother’s and Her Baby’s Health; Rosalind Press: London, UK, 2004; pp. 256–300. [Google Scholar]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Yeast biology. In Brewing: Science and Practice, 1st ed.; Briggs, D.E., Boulton, C.A., Brookes, P.A., Stevens, R., Eds.; Woodhead Publishing: Cambridge, UK, 2004; pp. 363–400. [Google Scholar]
- Johnson, E.A.; Echavarri-Erasun, C. Yeasts biotechnology. In The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2011; pp. 22–44. [Google Scholar]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Mertz, W. Effects and metabolism of glucose tolerance factor. Nutr. Rev. 1975, 33, 129–135. [Google Scholar] [CrossRef]
- Hosseinzadeh, P.; Djazayery, A.; Mostafavi, S.-A.; Javanbakht, M.H.; Derakhshanian, H.; Rahimiforoushani, A.; Djalali, M. Brewer’s yeast improves blood pressure in type 2 diabetes mellitus. Iran. J. Public Health 2013, 42, 602–609. [Google Scholar]
- Khosravi-Boroujeni, H.; Rostami, A.; Ravanshad, S.; Esmaillzadeh, A. Favorable effects on metabolic risk factors with daily brewer’s yeast in type 2 diabetic patients with hypercholesterolemia: A semi-experimental study. J. Diabetes 2012, 4, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.V.; Phung, O.J. Effect of chromium supplementation on glycated hemoglobin and fasting plasma glucose in patients with diabetes mellitus. Nutr. J. 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. Β-1, 3/1, 6-glucans and immunity: State of the art and future directions. Mol. Nutr. Food Res. 2020, 65, 1901071. [Google Scholar] [CrossRef]
- Samuelsen, A.B.C.; Schrezenmeir, J.; Knutsen, S.H. Effects of orally administered yeast-derived beta-glucans: A review. Mol. Nutr. Food Res. 2013, 58, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bekatorou, A.; Psarianos, C.; Koutinas, A.A. Production of food grade yeasts. Food Technol. Biotech. 2006, 44, 407–415. [Google Scholar]
- Halász, A.; Lásztity, R. Use of Yeast Biomass in Food Production. In Use of Yeast Biomass in Food Production; CRC Press: Boca Raton, FL, USA, 2017; pp. 45–113. [Google Scholar]
- US Food and Drug Administration. Code of Federal Regulations Title 21, Part 172–Food Additives Permitted for Direct Addition to Food for Human Consumption; Subpart I—Multipurpose Additives; Section 172.896—Dried Yeasts. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=dec9ed8dedde234ef14e7229a29cdffa&mc=true&node=se21.3.172_1896&rgn=div8 (accessed on 8 October 2020).
- Bahijiri, S.M.; Mira, S.A.; Mufti, A.M.; Ajabnoor, M.A. The effects of inorganic chromium and brewer’s yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with type 2 diabetes. Saudi Med. J. 2000, 21, 831–837. [Google Scholar]
- US Department of Agriculture. Technical Evaluation Report Compiled by OMRI for the USDA National Organic Program. Available online: https://www.ams.usda.gov/sites/default/files/media/Yeast%20TR%20Handling%201-22-14%20final.pdf (accessed on 2 June 2020).
- Alianmoghaddam, N.; Phibbs, S.; Benn, C. “I did a lot of Googling”: A qualitative study of exclusive breastfeeding support through social media. Women Birth 2019, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Government. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. Available online: https://www.nrv.gov.au/nutrients (accessed on 20 November 2020).
- Liu, J.; Zhang, B.; He, X.; Zhang, P.; Chai, Z. Selection of a high-biomass, chromium-rich yeast strain and optimization of cultivation conditions. J. Ind. Microbiol. Biotechnol. 2001, 27, 195–198. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission on selenium-enriched yeast as source for selenium. EFSA J. 2008, 766, 1–42. [Google Scholar]
- European Food Safety Authority. Scientific opinion on ChromoPrecise® cellular bound chromium yeast added for nutritional purposes as a source of chromium in food supplements and the bioavailability of chromium from this source. EFSA J. 2008, 6, 766. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Sajnaga, E.; Kozak, E.; Poleszak, E.; Malm, A. Dietary supplements based on the yeast biomass. Curr. Top. Nutraceutical Res. 2015, 13, 83–88. [Google Scholar]
- Winder, K. Lactation Cookies—90% of Our Fans Say this Recipe Works! Available online: https://www.bellybelly.com.au/breastfeeding/lactation-cookies/ (accessed on 4 June 2020).
- Karges, C. Best Lactation Cookies Recipe to Increase Breast Milk Supply Fast. Available online: https://www.crystalkarges.com/blog/family-friendly-lactation-oat-cookie-recipe (accessed on 4 June 2020).
- Desnoyers, M.; Giger-Reverdin, S.; Bertin, G.; Duvaux-Ponter, C.; Sauvant, D. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci. 2009, 92, 1620–1632. [Google Scholar] [CrossRef]
- Shurson, G. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed. Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Milewski, S.; Sobiec, P. Effect of dietary supplementation with Saccharomyces cerevisiae dried yeast on milk yield, blood biochemical and haematological indices in ewes. Bull. Vet. Inst. Pulawy 2009, 53, 753–758. [Google Scholar]
- Fortina, R.; Battaglini, L.; Opsi, F.; Tassone, S.; Renna, M.; Mimosi, A. Effects of inactivated yeast culture on rumen fermentation and performance of mid-lactation dairy cows. J. Anim. Vet. Adv. 2011, 10, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Ząbek, K.; Milewski, S.; Wójcik, R.; Siwicki, A.K. The effects of supplementing diets fed to pregnant and lactating ewes with Saccharomyces cerevisiae dried yeast. Turk. J. Vet. Anim. Sci. 2014, 38, 200–206. [Google Scholar] [CrossRef]
- Gomes, L.C.; Alcalde, C.R.; Macedo, F.D.A.F.D.; Dos Santos, G.T.; Valloto, A.A.; De Lima, L.S.; Molina, B.S.D.L. Performance of lactating goats fed diets containing inactive dry yeast. Rev. Bras. Zootec. 2012, 41, 2249–2254. [Google Scholar] [CrossRef] [Green Version]
- Dobicki, A.; Preś, J.; Zachwieja, A.; Kwaśnicki, R. Saccharomyces cerevisiae preparations in the feeding of cows and their effect on milk yield and composition as well as rumen microorganisms. Food Sci. Technol. 2006, 9, 13. [Google Scholar]
- Kuczaj, M.; Dobicki, A.; Pres, J.; Zachwieja, A.; Jakus, W. An influence of dried brewer’s yeast (Saccharomyces cerevisiae) addition before and after calving on yield and chemical composition of milk and biochemical indices of blood in the first 100 days of lactation. Electron. J. Pol Agric. Univ. 2010, 13, 12. [Google Scholar]
- Westland, A.; Martin, R.; White, R.; Martin, J.H. Mannan oligosaccharide prepartum supplementation: Effects on dairy cow colostrum quality and quantity. Animal 2017, 11, 1779–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, M.; Ohtsuka, H.; Izumi, K. Effect of yeast cell wall supplementation on production performances and blood biochemical indices of dairy cows in different lactation periods. Vet. World 2019, 12, 796–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ząbek, K.; Milewski, S.; Wójcik, R.; Siwicki, A.K. Effect of β-1,3/1,6-D-glucan in diet on productivity and humoral and cellular defense mechanisms in sheep. Acta Vet. Brno 2013, 82, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Zaleska, B.; Milewski, S.; Ząbek, K. Impact of Saccharomyces cerevisiae supplementation on reproductive performance, milk yield in ewes and offspring growth. Arch. Anim. Breed. 2015, 58, 79–83. [Google Scholar] [CrossRef] [Green Version]
- De Lima, L.S.; Alcalde, C.R.; Freitas, H.S.; Molina, B.S.D.L.; Macedo, F.D.A.F.D.; Horst, J.A. Performance of dairy goats fed diets with dry yeast from sugar cane as protein source. Rev. Bras. Zootec. 2012, 41, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Kang, K.; Piao, L.; Jeong, T.; Auclair, E.; Jonvel, S.; D’Inca, R.; Kim, Y.Y. Effects of live yeast supplementation to gestation and lactation diets on reproductive performance, immunological parameters and milk composition in sows. Livest. Sci. 2013, 152, 167–173. [Google Scholar] [CrossRef]
- Kim, S.W.; Brandherm, M.; Freeland, M.; Newton, B.; Cook, D.; Yoon, I. Effects of yeast culture supplementation to gestation and lactation diets on growth of nursing piglets. Asian Australas. J. Anim. Sci. 2008, 21, 1011–1014. [Google Scholar] [CrossRef]
- Shen, Y.B.; Carroll, J.A.; Yoon, I.; Mateo, R.D.; Kim, S.W. Effects of supplementing Saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets1,2. J. Anim. Sci. 2011, 89, 2462–2471. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Mokrzycka, A.; Pejsak, Z. Efficacy of mannanoligosaccharides additive to sows diets on colostrum, blood immunoglobulin content and production parameters of piglets. Pol. J. Vet. Sci. 2010, 13, 525–531. [Google Scholar] [PubMed]
- Szuba-Trznadel, A.; Rzasa, A.; Lira, R.; Fuchs, B. The influence of (1,3)-(1,6)-beta-D-glucan on the production results of sows and their offspring. J. Anim. Feed. Sci. 2014, 23, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Graugnard, D.; Samuel, R.; Xiao, R.; Spangler, L.; Brennan, K. Intestinal gene expression profiles of piglets benefit from maternal supplementation with a yeast mannan-rich fraction during gestation and lactation. Animal 2015, 9, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Chen, D.; Zheng, P.; Tian, G.; Wang, J.; Mao, X.; Yu, J.; He, J.; Li, B.; Huang, Z.; et al. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring. Anim. Feed. Sci. Technol. 2016, 218, 17–25. [Google Scholar] [CrossRef]
- Kuczaj, M.; Pres, J.; Zachwieja, A.; Twardon, J.; Orda, J.; Dobicki, A. Effect of supplementing dairy cows with live yeasts cells and dried brewer’s yeasts on milk chemical composition, somatic cell count and blood biochemical indices. Electron. J. Pol. Agric. Univ. 2014, 17, 06. [Google Scholar]
- Wojcik, R.; Milewski, S.; Malaczewska, J.; Tanski, Z.; Brzostowski, H.; Siwicki, A.K. Defence mechanisms of the offspring of ewes fed a diet supplemented with yeast (Saccharomyces cerevisiae) during pregnancy and lactation. Cent. Eur. J. Immunol. 2008, 33, 197–201. [Google Scholar]
- Wu, H.; Weng, B.; Chen, K.; Chiou, P.; Yu, B. Effect of dietary supplementation of β-1,3–1,6-glucan on reproductive performance and immunity of New Zealand White does and their pups. Livest. Sci. 2011, 135, 70–75. [Google Scholar] [CrossRef]
- Milewski, S.; Sobiech, P.; Ząbek, K.; Żarczyńska, K.; Antoszkiewicz, Z.; Wielgosz-Groth, Z. Effect of Saccharomyces cerevisiae yeast on milk protein content and composition and serum mineral concentrations in sheep. J. Elem. 2012, 17, 79–86. [Google Scholar] [CrossRef]
- Neville, M.C. Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 2993–3054. [Google Scholar]
- Holly, L.M.; Susan, J.M.; Peter, E.H. Evolution of lactation: Nutrition v. protection with special reference to five mammalian species. Nutr. Res. Rev. 2008, 21, 97–116. [Google Scholar]
- Stevens, C.E. Digestive System of Mammals; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M.P.L.V.O. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compost. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; Blackwell Science Ltd.: London, UK, 2001. [Google Scholar]
- Kaplan, B.J.; Crawford, S.G.; Field, C.J.; Simpson, J.S.A. Vitamins, minerals, and mood. Psychol. Bull. 2007, 133, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.; Liu, T.; Peter, I.; Buel, J.; Arsenault, L.; Scott, T.; Qiu, W.W. The homocysteine hypothesis of depression. Am. J. Psychiatry 2007, 164, 861–867. [Google Scholar] [CrossRef]
- Chong, M.F.; Wong, J.X.; Colega, M.; Chen, L.-W.; Van Dam, R.M.; Tan, C.S.; Lim, A.L.; Cai, S.; Broekman, B.F.; Lee, Y.S.; et al. Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: The GUSTO study. J. Psychiatr. Res. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Yokoyama, T.; Ohya, Y.; Fukushima, W.; Saito, K.; Ohfuji, S.; Kiyohara, C.; Hirota, Y. Dietary folate and vitamins B12, B6, and B2 intake and the risk of postpartum depression in Japan: The Osaka Maternal and Child Health Study. J. Affect. Disord. 2006, 96, 133–138. [Google Scholar] [CrossRef]
- Paoletti, A.M.; Orrù, M.M.; Marotto, M.F.; Pilloni, M.; Zedda, P.; Fais, M.F.; Piras, B.; Piano, C.; Pala, S.; Lello, S.; et al. Observational study on the efficacy of the supplementation with a preparation with several minerals and vitamins in improving mood and behaviour of healthy puerperal women. Gynecol. Endocrinol. 2013, 29, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Hallam, K.; Stojanovska, L.; Apostolopoulos, V. Yeast based spreads improve anxiety and stress. J. Funct. Foods 2018, 40, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Talbott, S.M.; Talbott, J.A. Baker’s yeast beta-glucan supplement reduces upper respiratory symptoms and improves mood state in stressed women. J. Am. Coll. Nutr. 2012, 31, 295–300. [Google Scholar] [CrossRef]
- Mah, B.L. Oxytocin, postnatal depression, and parenting: A systematic review. Harv. Rev. Psychiatry 2016, 24, 1–13. [Google Scholar] [CrossRef]
- Massey, S.H.; Backes, K.A.; Schuette, S.A. Plasma oxytocin concentration and depressive symptoms: A review of current evi-dence and directions for future research. Depress. Anxiety 2016, 33, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Hafeez, A.; Bilal, R.; Sikander, S.; Malik, A.; Minhas, F.; Tomenson, B.; Creed, F. The impact of perinatal depression on exclusive breastfeeding: A cohort study. Matern. Child Nutr. 2016, 12, 452–462. [Google Scholar] [CrossRef]
- Ueda, T.; Yokoyama, Y.; Irahara, M.; Aono, T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obstet. Gynecol. 1994, 84, 259–262. [Google Scholar] [PubMed]
- Stuebe, A.M.; Grewen, K.; Meltzer-Brody, S. Association between maternal mood and oxytocin response to breastfeeding. J. Women’s Health 2013, 22, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Whitley, J.; Wouk, K.; Bauer, A.E.; Grewen, K.; Gottfredson, N.C.; Meltzer-Brody, S.; Propper, C.; Mills-Koonce, R.; Pearson, B.; Stuebe, A. Oxytocin during breastfeeding and maternal mood symptoms. Psychoneuroendocrinology 2020, 113, 104581. [Google Scholar] [CrossRef]
- Peng, Z.; Qiao, W.; Wang, Z.; Dai, Q.; He, J.; Guo, C.; Xu, J.; Zhou, A. Chromium improves protein deposition through regulating the mRNA Levels of IGF-1, IGF-1R, and Ub in Rat Skeletal Muscle Cells. Biol. Trace Elem. Res. 2009, 137, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Liguori, S.A.; Fissore, M.F.; Oggero, R. Breast milk hormones and their protective effect on obesity. Int. J. Pediatr. Endocrinol. 2009, 1, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosser, C.G. Insulin-like growth factors in milk and mammary gland. J. Mammary Gland. Biol. Neoplasia 1996, 1, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Baumrucker, C.; Blum, J. Secretion of insulin-like growth factors in milk and their effect on the neonate. Livest. Prod. Sci. 1993, 35, 49–72. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Ghayour-Mobarhan, M.; Rooki, H.; Vakili, R.; Hashemy, S.I.; Mirhafez, S.R.; Shakeri, M.-T.; Kashanifar, R.; Pourbafarani, R.; Mirzaei, H.; et al. Comparative measurement of ghrelin, leptin, adiponectin, EGF and IGF-1 in breast milk of mothers with overweight/obese and normal-weight infants. Eur. J. Clin. Nutr. 2014, 69, 614–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kon, I.Y.; Shilina, N.M.; Gmoshinskaya, M.V.; Ivanushkina, T.A. The study of breast Milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann. Nutr. Metab. 2014, 65, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, H.; Delfi, L.; Rasouli, Y. The effect of β-glucan on prolactin secretion in gh3/b6 cells. Iran. J. Sci. Technol. Trans. A Sci. 2007, 31, 223–229. [Google Scholar]
- Williams, R.; Dias, D.A.; Jayasinghe, N.; Roessner, U.; Bennett, L.E. Beta-glucan-depleted, glycopeptide-rich extracts from Brewer’s and Baker’s yeast (Saccharomyces cerevisiae) lower interferon-gamma production by stimulated human blood cells in vitro. Food Chem. 2016, 197, 761–768. [Google Scholar] [CrossRef]
- Kankkunen, P.; Teirilä, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-β-Glucans Activate both dectin-1 and nlrp3 inflammasome in human macrophages. J. Immunol. 2010, 184, 6335–6342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetvicka, V.; Vashishta, A.; Saraswat-Ohri, S.; Vetvickova, J. Immunological effects of yeast-and mushroom-derived β-glucans. J. Med. Food 2008, 11, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Porsani, M.Y.; Paludetti, M.; Orlando, D.R.; Peconick, A.P.; Costa, R.C.; Oliveira, L.E.; Zangeronimo, M.G.; Sousa, R.V. Pro-tective effect of β-glucan and glutamine on intestinal and immunological damage in mice induced by cytarabine (Ara-C). Pesqui. Vet. Bras. 2017, 37, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, A.; Nieradka, R.; Kander, M.; Nowicki, M.; Wdowiak, M.; Kołodziejska-Sawerska, A. The effectiveness of natural and synthetic immunomodulators in the treatment of inflammatory bowel disease in dogs. Acta Vet. Hung. 2013, 61, 297–308. [Google Scholar] [CrossRef]
- Mosikanon, K.; Arthan, D.; Kettawan, A.; Tungtrongchitr, R.; Prangthip, P. Yeast β–glucan modulates inflammation and waist circumference in overweight and obese subjects. J. Diet. Suppl. 2016, 14, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Boyle, R.J.; Warner, J.O. Factors affecting breast milk composition and potential consequences for development of the allergic phenotype. Clin. Exp. Allergy 2014, 45, 583–601. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Yuan, J.; Zhang, B. Effect of Dietary 棺-1,3/1,6-glucan Supplementation on growth performance, immune response and plasma prostaglandin E2, growth hormone and ghrelin in weanling piglets. Asian Australas. J. Anim. Sci. 2008, 21, 707–714. [Google Scholar] [CrossRef]
- Richter, J.; Závorková, M.; Vetvicka, V.; Liehneová, I.; Kral, V.; Dobiasova, L.R. Effects of β-glucan and vitamin D supplementation on inflammatory parameters in patients with diabetic retinopathy. J. Diet. Suppl. 2018, 16, 369–378. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Giannì, M.L.; Morniroli, D.; Leone, L.; Roggero, P.; Agostoni, C.; De Cosmi, V.; Mosca, F. Hormones in breast milk and effect on infants’ growth: A systematic review. Nutrients 2019, 11, 1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, D.L.; Wilbey, R.A.; Grandison, A.S.; Roseiro, L.B. Milk oligosaccharides: A review. Int. J. Dairy Technol. 2015, 68, 305–321. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, J.M.; Arnold, C.; Ashorn, P.; Ashorn, U.; Chaima, D.; Cheung, Y.B.; Davis, J.C.; Fan, Y.-M.; Goonatilleke, E.; Kortekangas, E.; et al. Lipid-based nutrient supplements during pregnancy and lactation did not affect human milk oligosaccharides and bioactive proteins in a randomized trial. J. Nutr. 2017, 147, 1867–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.M.; Mohammad, M.; Bode, L.; Chu, D.M.; Ma, J.; Haymond, M.; Aagaard, K. 20: Maternal diet structures the breast milk microbiome in association with human milk oligosaccharides and gut-associated bacteria. Am. J. Obstet. Gynecol. 2017, 216, S15. [Google Scholar] [CrossRef]
- Hallam, M.C.; Barile, D.; Meyrand, M.; German, J.B.; Reimer, R.A. Maternal high-protein or high-prebiotic-fiber diets affect maternal milk composition and gut microbiota in rat dams and their offspring. Obesity 2014, 22, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M. Ochratoxin a in brewer’s yeast used as nutrient supplement. Mycotoxin Res. 2002, 18, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Biermaier, B.; Gross, M.; Schwaiger, K.; Gareis, M. Ochratoxin A in brewer’s yeast used as food supplement. Mycotoxin Res. 2015, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Novotna, E. Ochratoxin A: Developmental and reproductive toxicity—An Overview. Birth Defects Res. B Dev. Reprod. Toxicol. 2013, 98, 493–502. [Google Scholar] [CrossRef]
- Piotrowska, M.; Masek, A. Saccharomyces cerevisiae cell wall components as tools for ochratoxin a decontamination. Toxins 2015, 7, 1151–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peraica, M.; Richter, D.; Rašić, D. Mycotoxicoses in children. Arch. Ind. Hyg. Toxicol. 2014, 65, 347–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, J.B.; Ruiz, M.-J.; Manyes, L.; Juan-García, A. Blood, breast milk and urine: Potential biomarkers of exposure and estimated daily intake of ochratoxin A: A review. Food Addit. Contam. Part A 2015, 33, 1–16. [Google Scholar] [CrossRef]
- Hassan, A.M.; Sheashaa, H.A.; Fattah, M.F.A.; Ibrahim, A.Z.; Gaber, O.A.; Sobh, M.A. Study of ochratoxin A as an environmental risk that causes renal injury in breast-fed Egyptian infants. Pediatr. Nephrol. 2006, 21, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, K.; Blaszkewicz, M.; Campos, V.; Vega, M.; Degen, G.H. Exposure of infants to ochratoxin A with breast milk. Arch. Toxicol. 2013, 88, 837–846. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. PARNATE® (Tranylcypromine) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/012342s064lbl.pdf (accessed on 20 November 2020).
- McCabe-Sellers, B.J.; Staggs, C.G.; Bogle, M.L. Tyramine in foods and monoamine oxidase inhibitor drugs: A crossroad where medicine, nutrition, pharmacy, and food industry converge. J. Food Compos. Anal. 2006, 19, S58–S65. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum biomarkers for inflammatory bowel disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Barclay, G.; McKenzie, H.; Pennington, J.; Parratt, D.; Pennington, C. The effect of dietary yeast on the activity of stable chronic Crohn’s disease. Scand. J. Gastroenterol. 1992, 27, 196–200. [Google Scholar] [CrossRef]
- Król, E.; Krejpcio, Z.; Byks, H.; Bogdański, P.; Pupek-Musialik, D. Effects of chromium brewer’s yeast supplementation on body mass, blood carbohydrates, and lipids and minerals in Type 2 diabetic patients. Biol. Trace Elem. Res. 2010, 143, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Kleefstra, N.; Houweling, S.T.; Jansman, F.G.; Groenier, K.H.; Gans, R.O.; Jong, B.M.-D.; Bakker, S.J.; Bilo, H.J. Chromium treatment has no effect in patients with poorly controlled, insulin-treated type 2 diabetes in an obese western population: A randomized, double-blind, placebo-controlled trial. Diabetes Care 2006, 29, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Racek, J.; Trefil, L.; Rajdl, D.; Mudrová, V.; Hunter, D.; Senft, V. Influence of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and markers of oxidative stress in subjects with Type 2 diabetes mellitus. Biol. Trace Elem. Res. 2006, 109, 215–230. [Google Scholar] [CrossRef]
- Yanni, A.E.; Stamataki, N.S.; Konstantopoulos, P.; Stoupaki, M.; Abeliatis, A.; Nikolakea, I.; Perrea, D.; Karathanos, V.T.; Tentolouris, N. Controlling type-2 diabetes by inclusion of Cr-enriched yeast bread in the daily dietary pattern: A randomized clinical trial. Eur. J. Nutr. 2016, 57, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Trafikowska, U.; Sobkowiak, E.; Butler, J.; Whanger, P.; Zachara, B. Organic and inorganic selenium supplementation to lac-tating mothers increase the blood and milk Se concentrations and Se intake by breast-fed infants. J. Trace Elem. Med. Biol. 1998, 12, 77–85. [Google Scholar] [CrossRef]
- Kumpulainen, J.; Salmenperä, L.; Siimes, M.A.; Koivistoinen, P.; Perheentupa, J. Selenium status of exclusively breast-fed infants as influenced by maternal organic or inorganic selenium supplementation. Am. J. Clin. Nutr. 1985, 42, 829–835. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Burgert, S.L.; Milner, J.A.; Glass, L.; Kummer, R.; Deering, R.; Boucek, R.; Picciano, M.F. Selenium status of lactating women is affected by the form of selenium consumed. Am. J. Clin. Nutr. 1993, 58, 649–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trafikowska, U.; Zachara, B.; Wiacek, M.; Sobkowiak, E.; Czerwionka-Szaflarska, M. Selenium supply and glutathione peroxidase activity in breastfed Polish infants. Acta Paediatr. 1996, 85, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Moat, A.G. nicotinic acid biosynthesis in prototrophs and tryptophan auxotrophs of Saccharomyces cerevisiae. J. Biol. Chem. 1966, 241, 775–780. [Google Scholar] [CrossRef]
- Ministry of Primary Industries. Dietary Exposure to Ochratoxin A and Trichothecene Mycotoxins: Risk Estimates and Proportionality of Exposure source. Available online: http://www.mpi.govt.nz/dmsdocument/12930-dietary-exposure-to-ochratoxin-a-and-trichothecene-mycotoxins-risk-estimates-and-proportionality-of-exposure-source (accessed on 20 November 2020).
- World Health Organization. Food Additives Series 59 Safety Evaluation of Certain Food Additives and Contaminants. Available online: https://apps.who.int/iris/bitstream/handle/10665/43823/9789241660594_eng.pdf?sequence=1&isAllowed=y (accessed on 20 November 2020).

| Product Name | Production Information | Dose (g/Day) ** |
|---|---|---|
| Product 1: Brewer’s yeast powder 1 | Species and strains: Saccharomyces cerevisiae Medium: unclear Other ***: “brewer’s yeast is generally from the fermentation of beer, adding grains (such as rice or wheat), malt, dried flowers of hops to the medium for cultivation” | 30 |
| Product 2: Brewer’s yeast powder 2 | Species and strains: select strains of Saccharomyces cerevisiae Medium: sugar beet molasses | 30 |
| Product 3: Brewer’s yeast powder 3 | Species and strains: Saccharomyces cerevisiae Medium: malted barley in the production of beer Other ***: “debittered” | 15 |
| Product 4: Nutritional yeast flakes 4 | Species and strains: select strains of Saccharomyces cerevisiae Medium: enriched purified cane and beet molasses Other ***: “added niacin, pyridoxine hydrochloride, riboflavin, thiamin hydrochloride, folic acid and vitamin B12/ not from brewing process” | 9 |
| Product 5: Nutritional yeast flakes 5 | Species and strains: Saccharomyces cerevisiae Medium: a mixture of sugar cane and beet molasses Other ***: “NOT brewer’s yeast, baker’s yeast or torula yeast” | 15 |
| Product 6: Nutritional yeast flakes 6 | Species and strains: Saccharomyces cerevisiae Medium: molasses Other ***: “added niacin, pyridoxine HCl, riboflavin, thiamine HCl, folic acid and vitamin B12; gluten free” | 20 |
| Product 7: Brewer’s yeast powder 7 | Species and strains: no information Medium: no information Other ***: “from brewing process” | 11.5 |
| Product 8: Brewer’s yeast powder 8 | Species and strains: Saccharomyces cerevisiae Medium: no information Other ***: “from production of beer” | 16 |
| Product 9: Brewer’s yeast tablet 9 | Species and strains: Saccharomyces cerevisiae Medium: no information Other ***: “manufactured using non-debittered brewer’s yeast powder” | 1.8–3.6 (300 mg/tablet; 6–12 tablets/day) |
| Product 10: Brewer’s yeast tablet 10 | Species and strains: no information Medium: no information | 0.5–2 (500 mg/tablet; 1–4 tablets/day) |
| Nutrients | Content * µg/g Dry Yeast | ||||
|---|---|---|---|---|---|
| Product 1 * Brewer’s Yeast Powder | Product 2 * Brewer’s Yeast Powder | Product 3 * Brewer’s Yeast Powder | Product 4 * Nutritional Yeast Flakes | Product 5 * Nutritional Yeast Flakes | |
| Thiamin | 10 | 30 | 20 | 666.7 | 1600 |
| Riboflavin | 30 | 60 | 60 | 666.7 | 5 |
| Niacin | 190 | 333.3 | 380 | 3555.6 | 1000 |
| Vitamin B6 | 5 | 30 | 10 | 666.7 | 666.7 |
| Vitamin B12 | - | - | - | 1.6 | - |
| Folate (DFE) | 11.4 ** | 14.2 | 13.3 | 75 ** | - |
| Pantothenic acid | - | 100 | - | - | 2333.3 |
| Biotin | - | 0.3 | 0.3 | - | 1 |
| Calcium | - | 1500 | 733.3 | 666.7 | 1733.3 |
| Iron | 20 | 40 | 40 | 55.6 | 466.7 |
| Zinc | - | 166.7 | - | - | 2000 |
| Selenium | - | 2.2 | - | - | 1.4 |
| Chromium | - | 0.43 | - | - | 0.3 |
| Author or Source | Product Information | Dose (g/Day) | Format of the Supplement | Ingestion Method | Claimed Benefits |
|---|---|---|---|---|---|
| Anne Smith, IBCLC 1 | Brewer’s yeast | 2.7 (300 mg tablet) or 4.5 (500 mg tablet) ** | Tablets | 3 tablets taken with meals, 3 times per day | Increase milk production, contains B vitamins |
| Donna Murray, RN Reviewed by Meredith Shur, MD 2 | Brewer’s yeast | No information | Tablets or powder | No information | Increase milk supply, improve mood and baby blues |
| Rohit Garoo, BSc. Reviewed by Briana Violand, IBCLC 3 | Brewer’s yeast (used in brewing and making bread, but different from baker’s yeast) | 30 g *** | Recommend using powder because the dose of tablets varies between manufacturers | Add to cookies or water, 3 tablespoons per day, can increase the quantity by half-a-teaspoon a day if not seeing any improvement | Anecdotally increases milk supply, improves acne, improves glucose tolerance in diabetes, considered as a nutritional supplement for B vitamins and selenium |
| Kelly Winder, doula 4 | Brewer’s yeast (not substitutable with baker’s yeast or nutritional yeast) | Unclear **** | Powder or flakes | As an ingredient in lactation cookie recipe, 1 to 2 tablespoons per recipe, 2–5 cookies per day | Boost breast milk supply |
| Medela, breast pump manufacturer 5 | Brewer’s yeast | Unclear **** | Powder | As an ingredient in lactation cookie recipe, 5 tablespoons per recipe, no information of how many cookies to take per day | Increase breast milk supply |
| Crystal Karges, RDN, IBCLC 6 | Brewer’s yeast (can be substituted by nutritional yeast) | Unclear **** | Powder | As an ingredient in lactation cookie, 4 tablespoons per recipe, 2 cookies per day | Naturally help support milk supply, offer a boost of B vitamins, iron and other minerals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.L.; Brough, L.; Weber, J.L. Saccharomyces cerevisiae Yeast-Based Supplementation as a Galactagogue in Breastfeeding Women? A Review of Evidence from Animal and Human Studies. Nutrients 2021, 13, 727. https://doi.org/10.3390/nu13030727
Jia LL, Brough L, Weber JL. Saccharomyces cerevisiae Yeast-Based Supplementation as a Galactagogue in Breastfeeding Women? A Review of Evidence from Animal and Human Studies. Nutrients. 2021; 13(3):727. https://doi.org/10.3390/nu13030727
Chicago/Turabian StyleJia, Lili Lily, Louise Brough, and Janet Louise Weber. 2021. "Saccharomyces cerevisiae Yeast-Based Supplementation as a Galactagogue in Breastfeeding Women? A Review of Evidence from Animal and Human Studies" Nutrients 13, no. 3: 727. https://doi.org/10.3390/nu13030727
APA StyleJia, L. L., Brough, L., & Weber, J. L. (2021). Saccharomyces cerevisiae Yeast-Based Supplementation as a Galactagogue in Breastfeeding Women? A Review of Evidence from Animal and Human Studies. Nutrients, 13(3), 727. https://doi.org/10.3390/nu13030727