Polyphenols as a Diet Therapy Concept for Endometriosis—Current Opinion and Future Perspectives
Abstract
:1. Introduction
2. Current Treatment
3. Nutritional and Dietary Aspects of Endometriosis
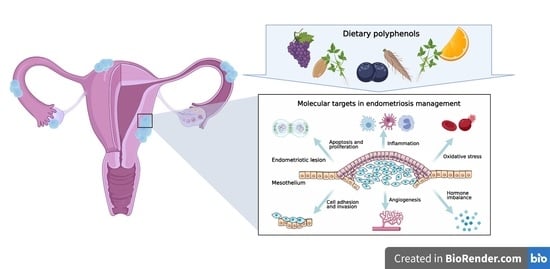
4. Molecular Targets in Endometriosis Dietary Management
4.1. Cell Survival and Apoptosis
4.2. Cell Attachment and Invasion
4.3. Angiogenesis
4.4. Immune Dysregulation
4.5. Oxidative Stress
4.6. Hormonal Imbalance
5. Potential of Polyphenol Compounds in Endometriosis Management
5.1. Flavonoids
5.1.1. Flavonols
Quercetin
5.1.2. Flavones
Apigenin
Baicalein
Wogonin
5.1.3. Isoflavonoids
Daidzein and Genistein
Puerarin
5.1.4. Flavanones
Naringenin
5.1.5. Chalcones
Xanthohumol
5.1.6. Flavanols
Epigallocatechin Gallate
5.2. Other Polyphenols
Curcumin
5.3. Phenolic Acids
Rosmarinic Acid
5.4. Stilbenes
Resveratrol
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.; Fernandez, M.L.; Anglesio, M.; Yong, P.J.; Carey, M.S. Endometriosis and endometriosis-associated cancers: New insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Kotani, Y.; Nakai, H.; Matsumura, N. Endometriosis-Associated Ovarian Cancer: The Origin and Targeted Therapy. Cancers 2020, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.; Tu, F.F.; Agarwal, S.K.; Chapron, C.; Soliman, A.M.; Chiuve, S.; Eichner, S.; Flores-Caldera, I.; Horne, A.W.; Kimball, A.B.; et al. Impact of Endometriosis on Life-Course Potential: A Narrative Review. Int. J. Gen. Med. 2021, ume 14, 9–25. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nicholes, K.; Shih, I.-M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 71–95. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Cao, L.; Xu, Z.; Ma, Y.; Liang, X. Anti-Angiogenic Alternative and Complementary Medicines for the Treatment of Endometriosis: A Review of Potential Molecular Mechanisms. Evidence-Based Complement. Altern. Med. 2018, 2018, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Alderman, M.H.; Tal, A.; Mamillapalli, R.; Coolidge, A.; Hufnagel, D.; Wang, Z.; Neisani, E.; Gidicsin, S.; Krikun, G.; et al. Hematogenous Dissemination of Mesenchymal Stem Cells from Endometriosis. Stem Cells 2018, 36, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Medina, M.G.; Lebovic, D.I. Endometriosis-associated nerve fibers and pain. Acta Obstet. Gynecol. Scand. 2009, 88, 968–975. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Chen, Y.-H.; Chang, H.-Y.; Au, H.-K.; Tzeng, C.-R.; Huang, Y.-H. Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies. Int. J. Mol. Sci. 2018, 19, 2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J. Med. Life 2014, 7, 349–357. [Google Scholar]
- Della Corte, L.; Noventa, M.; Ciebiera, M.; Magliarditi, M.; Sleiman, Z.; Karaman, E.; Catena, U.; Salvaggio, C.; Falzone, G.; Garzon, S. Phytotherapy in endometriosis: An up-to-date review. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Guo, S.-W. Recurrence of endometriosis and its control. Hum. Reprod. Updat. 2009, 15, 441–461. [Google Scholar] [CrossRef]
- Allen, C.; Hopewell, S.; Prentice, A.; Gregory, D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst. Rev. 2009, 2, CD004753. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.D.; Lang, S.A.; Kendziorski, J.A.; Sroga-Rios, J.M.; Herzog, T.J.; Burns, K.A. Endometriosis: Where are we and where are we going? Reproduction 2016, 152, R63–R78. [Google Scholar] [CrossRef] [Green Version]
- Gheorghisan-Galateanu, A.; Gheorghiu, M.L.; Gheorghisan-Galateanu, A. Hormonal Therapy in Women of Reproductive Age with Endometriosis: An Update. Acta Endocrinol. (Bucharest) 2019, 15, 276–281. [Google Scholar] [CrossRef]
- E Schindler, A. Dienogest in long-term treatment of endometriosis. Int. J. Women’s Health 2011, 3, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.R.; Martínez-Varea, A.; Hidalgo-Mora, J.J.; Pellicer, A. Pharmacologic therapies in endometriosis: A systematic review. Fertil. Steril. 2012, 98, 529–555. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz-Przondziono, J.; Lemm, M.; Kwiatkowska-Pamuła, A.; Ziółko, E.; Wójtowicz, M.K. Influence of diet on the risk of developing endometriosis. Ginekol. Polska 2017, 88, 96–102. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, W.; Li, T.; Zhao, Y.; Bu, H.; Song, S. Effects of acupuncture for the treatment of endometriosis-related pain: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186616. [Google Scholar] [CrossRef] [PubMed]
- Wattier, J.-M. Conventional analgesics and non-pharmacological multidisciplinary therapeutic treatment in endometriosis: CNGOF-HAS Endometriosis Guidelines. Gynecol. Obstet. Fertil. Senol. 2018, 46, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Osteen, K.G.; Bruner-Tran, K.L.; Lockwood, C.J.; Krikun, G.; Sokalska, A.; Duleba, A.J. Novel Therapies Targeting Endometriosis. Reprod. Sci. 2011, 18, 814–823. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, R.; Rowe, H.; Fisher, J. Self-management in condition-specific health: A systematic review of the evidence among women diagnosed with endometriosis. BMC Women’s Health 2019, 19, 1–19. [Google Scholar] [CrossRef]
- Armour, M.; Sinclair, J.; Chalmers, K.J.; Smith, C.A. Self-management strategies amongst Australian women with endometriosis: A national online survey. BMC Complement. Altern. Med. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Missmer, S.A.; Chavarro, J.E.; Malspeis, S.; Bertone-Johnson, E.R.; Hornstein, M.D.; Spiegelman, D.; Barbieri, R.L.; Willett, W.C.; Hankinson, S.E. A prospective study of dietary fat consumption and endometriosis risk. Hum. Reprod. 2010, 25, 1528–1535. [Google Scholar] [CrossRef] [Green Version]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.M.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y.-H.; Liu, C.-F.; Tsui, I.; Guo, Y.; Ai, B.-B.; Han, F.-J. The Complementary and Alternative Medicine for Endometriosis: A Review of Utilization and Mechanism. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Trabert, B.; Peters, U.; De Roos, A.J.; Scholes, D.; Holt, V.L. Diet and risk of endometriosis in a population-based case–control study. Br. J. Nutr. 2010, 105, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, L.D.G.C.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Vigano, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.K.; Arablou, T. Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms (Review). Biomed. Pharmacother. 2017, 91, 220–228. [Google Scholar] [CrossRef]
- Jiang, Q.-Y.; Wu, R.-J. Growth mechanisms of endometriotic cells in implanted places: A review. Gynecol. Endocrinol. 2012, 28, 562–567. [Google Scholar] [CrossRef]
- Laux-Biehlmann, A.; D’Hooghe, T.; Zollner, T.M. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol. Sci. 2015, 36, 270–276. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.-J.; et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, Y.; Taniguchi, F.; Nakamura, K.; Nagira, K.; Khine, Y.M.; Kiyama, T.; Uegaki, T.; Izawa, M.; Harada, T. Lipopolysaccharide promotes the development of murine endometriosis-like lesions via the nuclear factor-kappa B pathway. Am. J. Reprod. Immunol. 2017, 77, e12631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballester, M.; Gonin, J.; Rodenas, A.; Bernaudin, J.-F.; Rouzier, R.; Coutant, C.; Daraï, E. Eutopic endometrium and peritoneal, ovarian and colorectal endometriotic tissues express a different profile of Nectin-1, -3, -4 and nectin-like molecule 2. Hum. Reprod. 2012, 27, 3179–3186. [Google Scholar] [CrossRef] [Green Version]
- Agic, A.; Djalali, S.; Diedrich, K.; Hornung, D. Apoptosis in Endometriosis. Gynecol. Obstet. Investig. 2009, 68, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, F.; Kaponis, A.; Izawa, M.; Kiyama, T.; Deura, I.; Ito, M.; Iwabe, T.; Adonakis, G.; Terakawa, N.; Harada, T. Apoptosis and endometriosis. Front. Biosci. 2011, 3, 648–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbracia, M.; Valeri, C.; Antonini, G.; Biagiotti, G.; Pacchiarotti, A.; Pacchiarotti, A. Fas and Fas-Ligand in Eutopic and Ectopic Endometrium of Women With Endometriosis: The Possible Immune Privilege of Ectopic Endometrium. Reprod. Sci. 2016, 23, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yovich, J.L.; Rowlands, P.K.; Lingham, S.; Sillender, M.; Srinivasan, S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod. Biomed. Online 2020, 40, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.; Bhattacharya, P.; Das Mahapatra, P.; Swarnakar, S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J. Pineal Res. 2010, 49, 156–168. [Google Scholar] [CrossRef]
- Weusten, A.Y.D.; Groothuis, P.G.; Dunselman, G.A.; De Goeij, A.F.; Arends, J.W.; Evers, J.L. Morphological changes in mesothelial cells induced by shed menstrual endometrium in vitro are not primarily due to apoptosis or necrosis. Hum. Reprod. 2000, 15, 1462–1468. [Google Scholar] [CrossRef]
- Kyama, C.M.; Overbergh, L.; Mihalyi, A.; Meuleman, C.; Mwenda, J.M.; Mathieu, C.; D’Hooghe, T.M. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil. Steril. 2008, 89, 301–310. [Google Scholar] [CrossRef]
- Canis, M.; Bourdel, N.; Houlle, C.; Gremeau, A.-S.; Botchorishvili, R.; Matsuzaki, S. Trauma and endometriosis. A review. May we explain surgical phenotypes and natural history of the disease? J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 219–227. [Google Scholar] [CrossRef]
- Carr, B.R.; Arici, A.; Witz, C.A. Cell Adhesion Molecules and Endometriosis. Semin. Reprod. Med. 2003, 21, 173–182. [Google Scholar] [CrossRef]
- Rutherford, E.J.; Hill, A.D.K.; Hopkins, A.M. Adhesion in Physiological, Benign and Malignant Proliferative States of the Endometrium: Microenvironment and the Clinical Big Picture. Cells 2018, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Bałkowiec, M.; Maksym, R.B.; Włodarski, P.K. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (Review). Mol. Med. Rep. 2018, 18, 3123–3136. [Google Scholar] [CrossRef] [Green Version]
- Weigel, M.T.; Krämer, J.; Schem, C.; Wenners, A.; Alkatout, I.; Jonat, W.; Maass, N.; Mundhenke, C. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 74–78. [Google Scholar] [CrossRef]
- Bruner, K.L.; Eisenberg, E.; Gorstein, F.; Osteen, K.G. Progesterone and transforming growth factor-β coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis. Steroids 1999, 64, 648–653. [Google Scholar] [CrossRef]
- Rocha, A.L.L.; Reis, F.M.; Taylor, R.N. Angiogenesis and Endometriosis. Obstet. Gynecol. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Kianpour, M.; Nematbakhsh, M.; Ahmadi, S.M.; Jafarzadeh, M.; Hajjarian, M.; Pezeshki, Z.; Safari, T.; Eshraghi-Jazi, F. Se-rum and Peritoneal Fluid Levels of Vascular Endothelial Growth Factor in Women with Endometriosis. Int. J. Fertil. Steril. 2013, 7, 96–99. [Google Scholar] [PubMed]
- Laschke, M.; Elitzsch, A.; Vollmar, B.; Vajkoczy, P.; Menger, M. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum. Reprod. 2005, 21, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.M.; Rohwer, N.; Funakoshi, T.; Cramer, T.; Bernhardt, W.; Birsner, A.; Folkman, J.; D’Amato, R.J. 2-Methoxyestradiol Inhibits Hypoxia-Inducible Factor-1α and Suppresses Growth of Lesions in a Mouse Model of Endometriosis. Am. J. Pathol. 2008, 172, 534–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, D.E.; Abrao, M.S.; Berardo, P.T.; Takiya, C.M.; Nasciutti, L.E. Vascular density and distribution of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) are significantly higher in patients with deeply infiltrating endometriosis affecting the rectum. Fertil. Steril. 2008, 90, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rizner, T.L. Diagnostic potential of peritoneal fluid biomarkers of endometriosis. Expert Rev. Mol. Diagn. 2015, 15, 557–580. [Google Scholar] [CrossRef]
- Agic, A.; Xu, H.; Finas, D.; Banz, C.; Diedrich, K.; Hornung, D. Is Endometriosis Associated with Systemic Subclinical Inflammation? Gynecol. Obstet. Investig. 2006, 62, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Association of the Precursor of Interleukin-1β and Peritoneal Inflammation-Role in Pathogenesis of Endometriosis. J. Clin. Lab. Anal. 2016, 30, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Oosterlynck, D.J.; Meuleman, C.; Waer, M.; Koninckx, P.R.; Vandeputte, M. Immunosuppressive activity of peritoneal fluid in women with endometriosis. Obstet. Gynecol. 1993, 82, 206–212. [Google Scholar]
- Laganà, A.S.; Triolo, O.; Salmeri, F.M.; Granese, R.; Palmara, V.I.; Frangez, H.B.; Bokal, E.V.; Sofo, V. Natural Killer T cell subsets in eutopic and ectopic endometrium: A fresh look to a busy corner. Arch. Gynecol. Obstet. 2016, 293, 941–949. [Google Scholar] [CrossRef]
- Osuga, Y.; Koga, K.; Hirota, Y.; Hirata, T.; Yoshino, O.; Taketani, Y. Lymphocytes in Endometriosis. Am. J. Reprod. Immunol. 2010, 65, 1–10. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 2006, 13, 126–134. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Harlev, A.; Gupta, S.; Agarwal, A. Targeting oxidative stress to treat endometriosis. Expert Opin. Ther. Targets 2015, 19, 1447–1464. [Google Scholar] [CrossRef]
- Lousse, J.-C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci. 2012, E4, 23–40. [Google Scholar] [CrossRef]
- Mulgund, A.; Doshi, S.; Agarwal, A. Chapter 25—The Role of Oxidative Stress in Endometriosis. In Handbook of Fertility; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 273–281. ISBN 978-0-12-800872-0. [Google Scholar]
- Nagayasu, M.; Imanaka, S.; Kimura, M.; Maruyama, S.; Kobayashi, H. Nonhormonal Treatment for Endometriosis Focusing on Redox Imbalance. Gynecol. Obstet. Investig. 2021, 1–12. [Google Scholar] [CrossRef]
- Santanam, N.; Kavtaradze, N.; Murphy, A.; Dominguez, C.; Parthasarathy, S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl. Res. 2013, 161, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of Estrogen Receptor-β in Endometriosis. Nat. Med. 2012, 18, 1016–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Ito, F.; Koshiba, A.; Kataoka, H.; Tanaka, Y.; Okimura, H.; Khan, K.N.; Kitawaki, J. Aromatase as a target for treating endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 1673–1681. [Google Scholar] [CrossRef] [Green Version]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. The Genetic Background of Endometriosis: Can ESR2 and CYP19A1 Genes Be a Potential Risk Factor for Its Development? Int. J. Mol. Sci. 2020, 21, 8235. [Google Scholar] [CrossRef]
- Bulun, S.E.; Cheng, Y.-H.; Pavone, M.E.; Yin, P.; Imir, G.; Utsunomiya, H.; Thung, S.; Xue, Q.; Marsh, E.E.; Tokunaga, H.; et al. 17β-Hydroxysteroid Dehydrogenase-2 Deficiency and Progesterone Resistance in Endometriosis. Semin. Reprod. Med. 2010, 28, 044–050. [Google Scholar] [CrossRef] [Green Version]
- García-Gómez, E.; Vázquez-Martínez, E.R.; Reyes-Mayoral, C.; Cruz-Orozco, O.P.; Camacho-Arroyo, I.; Cerbón, M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front. Endocrinol. 2020, 10, 935. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, R.M.; Kim, T.H.; Shin, J.-H.; Jeong, J.-W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2016, 61. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Brzostek, T.; Gniadek, A.; Favari, C.; Mena, P.; Libra, M.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary phytoestrogens and biomarkers of their intake in relation to cancer survival and recurrence: A comprehensive systematic review with meta-analysis. Nutr. Rev. 2021, 79, 42–65. [Google Scholar] [CrossRef]
- Bhyan, S.B.; Zhao, L.; Wee, Y.; Liu, Y.; Zhao, M. Genetic links between endometriosis and cancers in women. PeerJ 2019, 7, e8135. [Google Scholar] [CrossRef] [PubMed]
- Taskin, O.; Rikhraj, K.; Tan, J.; Sedlak, T.; Rowe, T.C.; Bedaiwy, M.A. Link between Endometriosis, Atherosclerotic Cardiovascular Disease, and the Health of Women Midlife. J. Minim. Invasive Gynecol. 2019, 26, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose–Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, 2001019. [Google Scholar] [CrossRef] [PubMed]
- Wieser, F.; Cohen, M.; Gaeddert, A.; Yu, J.; Burks-Wicks, C.; Berga, S.L.; Taylor, R.N. Evolution of medical treatment for endometriosis: Back to the roots? Hum. Reprod. Updat. 2007, 13, 487–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flower, A.; Liu, J.P.; Chen, S.; Lewith, G.; Little, P. Chinese herbal medicine for endometriosis. Cochrane Database Syst. Rev. 2009, 3, CD006568. [Google Scholar] [CrossRef]
- Sesti, F.; Capozzolo, T.; Pietropolli, A.; Collalti, M.; Bollea, M.R.; Piccione, E. Dietary therapy: A new strategy for management of chronic pelvic pain. Nutr. Res. Rev. 2010, 24, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Whang, K.-Y.; Song, G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J. Nutr. Biochem. 2019, 63, 87–100. [Google Scholar] [CrossRef]
- Jamali, N.; Zal, F.; Mostafavi-Pour, Z.; Samare-Najaf, M.; Poordast, T.; Dehghanian, A. Ameliorative Effects of Quercetin and Metformin and Their Combination Against Experimental Endometriosis in Rats. Reprod. Sci. 2021, 28, 683–692. [Google Scholar] [CrossRef]
- Signorile, P.G.; Viceconte, R.; Baldi, A. Novel dietary supplement association reduces symptoms in endometriosis patients. J. Cell. Physiol. 2018, 233, 5920–5925. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J. Cell. Physiol. 2018, 233, 3055–3065. [Google Scholar] [CrossRef]
- Suou, K.; Taniguchi, F.; Tagashira, Y.; Kiyama, T.; Terakawa, N.; Harada, T. Apigenin inhibits tumor necrosis factor α–induced cell proliferation and prostaglandin E2 synthesis by inactivating NFκB in endometriotic stromal cells. Fertil. Steril. 2011, 95, 1518–1521. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Akkol, E.K. Promising activity of Anthemis austriaca Jacq. on the endometriosis rat model and isolation of its active constituents. Saudi Pharm. J. 2019, 27, 889–899. [Google Scholar] [CrossRef]
- Jin, Z.; Huang, J.; Zhu, Z. Baicalein reduces endometriosis by suppressing the viability of human endometrial stromal cells through the nuclear factor-κB pathway in vitro. Exp. Ther. Med. 2017, 14, 2992–2998. [Google Scholar] [CrossRef]
- Ke, J.; Yang, J.; Li, J.; Xu, Z.; Li, M.; Zhu, Z. Baicalein inhibits FURIN-MT1-MMP-mediated invasion of ectopic endometrial stromal cells in endometriosis possibly by reducing the secretion of TGFB1. Am. J. Reprod. Immunol. 2021, 85, 13344. [Google Scholar] [CrossRef]
- Ferella, L.; Bastón, J.I.; Bilotas, M.A.; Singla, J.J.; González, A.M.; Olivares, C.N.; Meresman, G.F. Active compounds present in Rosmarinus officinalis leaves and Scutellaria baicalensis root evaluated as new therapeutic agents for endometriosis. Reprod. Biomed. Online 2018, 37, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, E.; Oktem, M.; Esinler, I.; Toru, S.A.; Zeyneloglu, H.B. Genistein causes regression of endometriotic implants in the rat model. Fertil. Steril. 2007, 88, 1129–1134. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Miura, T.; Hanaoka, T.; Iwasaki, M.; Sasaki, H.; Tanaka, T.; Nakao, H.; Katoh, T.; Ikenoue, T.; Kabuto, M.; et al. Effect of Soy Isoflavones on Endometriosis: Interaction with Estrogen Receptor 2 Gene Polymorphism. Epidemiology 2007, 18, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Han, J.; Zai, D.; Ji, M.; Cheng, W.; Xu, L.; Yang, L.; He, M.; Ni, J.; et al. Puerarin Suppresses Invasion and Vascularization of Endometriosis Tissue Stimulated by 17β-Estradiol. PLoS ONE 2011, 6, e25011. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Liu, Y.; Yang, S.; Zhai, D.; Zhang, D.; Bai, L.; Wang, Z.; Yu, J.; Yu, C.; Cai, Z. Puerarin suppresses proliferation of endometriotic stromal cells in part via differential recruitment of nuclear receptor coregulators to estrogen receptor-α. J. Steroid Biochem. Mol. Biol. 2013, 138, 421–426. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, L.; Zhang, D.; Zhai, D.; Shen, W.; Bai, L.; Liu, Y.; Cai, Z.; Li, J.; Yu, C. The Effects and Possible Mechanisms of Puerarin to Treat Endometriosis Model Rats. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef]
- Rudzitis-Auth, J.; Körbel, C.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum. Reprod. 2012, 27, 1735–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Man, G.C.W.; Wang, C.C. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021.e1–1028.e1. [Google Scholar] [CrossRef]
- Wang, C.C.; Xu, H.; Man, G.C.W.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.Y.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2012, 16, 59–69. [Google Scholar] [CrossRef]
- Kim, K.-H.; Na Lee, E.; Park, J.K.; Lee, J.-R.; Kim, J.-H.; Choi, H.-J.; Kim, B.-S.; Lee, H.-W.; Lee, K.-S.; Yoon, S. Curcumin Attenuates TNF-α-induced Expression of Intercellular Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1 and Proinflammatory Cytokines in Human Endometriotic Stromal Cells. Phytotherapy Res. 2012, 26, 1037–1047. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Yu, Z.; Peng, H.-Y.; Zhang, C.-J. Curcumin Inhibits Endometriosis Endometrial Cells by Reducing Estra-diol Production. Iran. J. Reprod. Med. 2013, 11, 415–422. [Google Scholar]
- Cao, H.; Wei, Y.-X.; Zhou, Q.; Zhang, Y.; Guo, X.-P.; Zhang, J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol. Med. Rep. 2017, 16, 5611–5617. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, I.; Banerjee, S.; Driss, A.; Xu, W.; Mehrabi, S.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J. Cell. Physiol. 2019, 234, 6298–6312. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012, 83, 797–804. [Google Scholar] [CrossRef]
- Swarnakar, S.; Paul, S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J. Biochem. Biophys. 2009, 46, 59–65. [Google Scholar]
- Zhang, C.-J.; Hu, Y.-Y.; Zhang, Y.; Cao, H.; Wang, H. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int. J. Mol. Med. 2010, 27, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Bin, L. Estrogen-Independent Inhibition of Curcumin on Formation of Endometriotic Foci in Rats with Experimental Endo-metriosis. Med. J. West China 2012, 5. [Google Scholar]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol Inhibits Development of Experimental Endometriosis In Vivo and Reduces Endometrial Stromal Cell Invasiveness In Vitro1. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Amaya, S.C.; Savaris, R.F.; Filipovic, C.J.; Wise, J.D.; Hestermann, E.; Young, S.L.; Lessey, B.A. Resveratrol and Endometrium: A Closer Look at an Active Ingredient of Red Wine Using in Vivo and in Vitro Models. Reprod. Sci. 2014, 21, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Olivares, C.; Bilotas, M.; Bastón, J.; Singla, J.; Meresman, G.; Barañao, R. Natural therapies assessment for the treatment of endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arablou, T.; Delbandi, A.; Khodaverdi, S.; Arefi, S.; Kolahdouz-Mohammadi, R.; Heidari, S.; Mohammadi, T.; Aryaeian, N. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother. Res. 2019, 33, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.R.; Rashidi, Z.; Chobsaz, F.; Niromand, E.; Khazaei, M. Inhibitory effect of resveratrol on the growth and angiogenesis of human endometrial tissue in an In Vitro three-dimensional model of endometriosis. Reprod. Biol. 2020, 20, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz-Mohammadi, R.; Shidfar, F.; Khodaverdi, S.; Arablou, T.; Heidari, S.; Rashidi, N.; Delbandi, A. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J. Cell. Mol. Med. 2021, 25, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Ergenoğlu, A.M.; Yeniel, A. Özgür; Erbaş, O.; Aktuğ, H.; Yildirim, N.; Ulukuş, M.; Taskiran, D. Regression of Endometrial Implants by Resveratrol in an Experimentally Induced Endometriosis Model in Rats. Reprod. Sci. 2013, 20, 1230–1236. [Google Scholar] [CrossRef]
- Cenksoy, P.O.; Oktem, M.; Erdem, O.; Karakaya, C.; Cenksoy, C.; Erdem, A.; Guner, H.; Karabacak, O. A potential novel treatment strategy: Inhibition of angiogenesis and inflammation by resveratrol for regression of endometriosis in an experimental rat model. Gynecol. Endocrinol. 2015, 31, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; E Aydin, N.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 2014, 10, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, X.; Zhou, L.; Zhu, M.; Yao, S.; Ding, Y.; Liu, T.; Wang, Y.; Zhang, Y.; Li, R.; et al. MTA1, a Target of Resveratrol, Promotes Epithelial-Mesenchymal Transition of Endometriosis via ZEB2. Mol. Ther. Methods Clin. Dev. 2020, 19, 295–306. [Google Scholar] [CrossRef]
- Maia, H.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int. J. Women’s Health 2012, 4, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Nashtaei, M.S.; Pazhohan, A.; Bahramrezai, M.; Berenjian, S.; Sobhani, A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: A randomized exploratory trial. Gynecol. Endocrinol. 2019, 35, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-Mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfandyari, S.; Sobhani, A. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J. Reprod. Immunol. 2021, 143, 103248. [Google Scholar] [CrossRef] [PubMed]
- Meresman, G.F.; Götte, M.; Laschke, M.W. Plants as source of new therapies for endometriosis: A review of preclinical and clinical studies. Hum. Reprod. Updat. 2021, 27, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.O.; Leem, K.; Park, J.; Lee, P.; Ahn, D.-K.; Lee, B.C.; Park, H.K.; Suk, K.; Kim, S.Y.; Kim, H. Cytoprotective effect of Scutellaria baicalensis in CA1 hippocampal neurons of rats after global cerebral ischemia. J. Ethnopharmacol. 2001, 77, 183–188. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The Fascinating Effects of Baicalein on Cancer: A Review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef] [Green Version]
- Po, L.S.; Chen, Z.-Y.; Tsang, D.S.C.; Leung, L.K. Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells. Cancer Lett. 2002, 187, 33–40. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Q.; Li, K.; Zhao, H.; Han, Z.; Li, F.; Sun, M.; Zhang, Y. Antitumor activity of baicalein on the mice bearing U14 cervical cancer. Afr. J. Biotechnol. 2011, 10, 14169–14176. [Google Scholar] [CrossRef] [Green Version]
- Waisundara, V.Y.; Siu, S.Y.; Hsu, A.; Huang, D.; Tan, B.K. Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci. 2011, 88, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xia, J.; Yang, H.; Li, Y.; Liu, S.; Cao, Y.; Tang, L.; Yu, X. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/β-catenin signaling pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Limer, J.L.; Speirs, V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. 2004, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Murphy, P.A. Isoflavone Content in Commercial Soybean Foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Wagner, J.D.; Anthony, M.S.; Cline, J.M. Soy Phytoestrogens: Research on Benefits and Risks. Clin. Obstet. Gynecol. 2001, 44, 843–852. [Google Scholar] [CrossRef]
- Takaoka, O.; Mori, T.; Ito, F.; Okimura, H.; Kataoka, H.; Tanaka, Y.; Koshiba, A.; Kusuki, I.; Shigehiro, S.; Amami, T.; et al. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J. Steroid Biochem. Mol. Biol. 2018, 181, 125–132. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Puerarin: A Review of Pharmacological Effects. Phytotherapy Res. 2014, 28, 961–975. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Jeong, H.G. Mechanism of phytoestrogen puerarin-mediated cytoprotection following oxidative injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol. Appl. Pharmacol. 2008, 233, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. HL-60 differentiating activity and flavonoid content of the readily extractable fraction prepared from citrus juices. J. Agric. Food Chem. 1998, 47, 128–135. [Google Scholar] [CrossRef]
- Bao, L.; Liu, F.; Guo, H.-B.; Li, Y.; Tan, B.-B.; Zhang, W.-X.; Peng, Y.-H. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumor Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-Induced Apoptotic Cell Death in Prostate Cancer Cells Is Mediated via the PI3K/AKT and MAPK Signaling Pathways. J. Cell. Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Ascenzi, P.; Bulzomi, P.; Marino, M. The Nutritional Flavanone Naringenin Triggers Antiestrogenic Effects by Regulating Estrogen Receptor α-Palmitoylation. Endocrinology 2008, 149, 2567–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as Adjunctive with Conventional Anticancer Therapies. Curr. Pharm. Des. 2016, 22, 4201–4218. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chakravarty, B.; Chaudhury, K. Nanoparticle-Assisted Combinatorial Therapy for Effective Treatment of Endometriosis. J. Biomed. Nanotechnol. 2015, 11, 789–804. [Google Scholar] [CrossRef]
- Chen, D.; Wan, S.B.; Yang, H.; Yuan, J.; Chan, T.H.; Dou, Q.P. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Advances in Clinical Chemistry 2011, 53, 155–177. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lui, W.; Chu, C.; Ng, P.; Wang, C.; Rogers, M. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum. Reprod. 2008, 24, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Hack, M.E.A.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Siemann, E.H.; Creasy, L.L. Concentration of the Phytoalexin Resveratrol in Wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Nakata, R.; Takahashi, S.; Inoue, H. Recent Advances in the Study on Resveratrol. Biol. Pharm. Bull. 2012, 35, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Chao, S.-C.; Chen, Y.-J.; Huang, K.-Y.; Kuo, K.-L.; Yang, T.-H.; Wang, C.-C.; Tang, C.-H.; Yang, R.-S.; Liu, S.-H. Induction of sirtuin-1 signaling by resveratrol induces human chondrosarcoma cell apoptosis and exhibits antitumor activity. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Dull, A.-M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilhan, M.; Dereli, F.T.G.; Akkol, E.K.; Gürağaç, F.T. Novel Drug Targets with Traditional Herbal Medicines for Overcoming Endometriosis. Curr. Drug Deliv. 2019, 16, 386–399. [Google Scholar] [CrossRef]
- da Silva, D.M.; Gross, L.A.; Neto, E.D.P.G.; Lessey, B.A.; Savaris, R.F. The Use of Resveratrol as an Adjuvant Treatment of Pain in Endometriosis: A Randomized Clinical Trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Yang, X.; Belosay, A.; Hartman, J.A.; Song, H.; Zhang, Y.; Wang, W.; Doerge, D.R.; Helferich, W.G. Dietary soy isoflavones increase metastasis to lungs in an experimental model of breast cancer with bone micro-tumors. Clin. Exp. Metastasis 2015, 32, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Carbonel, A.A.F.; Calió, M.L.; Santos, M.A.; Bertoncini, C.R.A.; Sasso, G.D.S.; Simões, R.S.; Simões, M.J.; Soares, J.M. Soybean isoflavones attenuate the expression of genes related to endometrial cancer risk. Climacteric 2014, 18, 389–398. [Google Scholar] [CrossRef]
- Zhong, X.-S.; Ge, J.; Chen, S.-W.; Xiong, Y.-Q.; Ma, S.-J.; Chen, Q. Association between Dietary Isoflavones in Soy and Legumes and Endometrial Cancer: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2018, 118, 637–651. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Qin, L.-Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2010, 125, 315–323. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food. Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 13, 4246. [Google Scholar] [CrossRef] [Green Version]

| Mechanisms of Action | Molecular Targets | Disease Model | Ref. |
|---|---|---|---|
| Quercetin | |||
| ↓Cell proliferation ↑Apoptosis | ↓Mitochondrial membrane potential, ↓ERK1/2, ↓p38 MAPK, ↓AKT, DNA fragmentation | Endometriosis cell lines | [89] |
| ↓Cell proliferation | ↓CCND1, ↓Cyclin D1 | Murine endometriosis model | [89] |
| ↓Cell proliferation, ↑Autophagy ↓Endometriotic lesion size ↓Oxidative stress | ↓Serum E2, ↓Serum TNF-α ↑NQO1 enzyme activity, mTOR inhibition ↑ NRF2, ↑BECN1, ↑ATG5 | Rat endometriosis model | [90] |
| ↓Inflammation ↓Endometriosis-related pain ↓Endometriotic lesion size | ↓Serum PGE2 ↓Serum CA-125 | 30 patients with IV endometriosis stage treated for 3 months with 200 mg quercetin | [91] |
| Apigenin | |||
| ↓Cell proliferation ↑Apoptosis | ERK1/2 and JNK inhibition, ↑BAX, ↑Cyt- c, ↑ROS, ↑ER stress, ↑Calcium ions in cytosol ↓Mitochondrial membrane potential | Endometriosis cell lines | [92] |
| ↓Cell proliferation ↓Inflammation | ↓COX-2, ↓PGE2, ↓IL-8 NF-κB inhibition | Primary endometriotic stromal cells from ovarian endometrioma | [93] |
| ↓Angiogenesis, ↓Inflammation ↓Endometriotic implant volume | ↓Peritoneal VEGF, ↓Peritoneal TNF-α ↓Peritoneal IL-6 | Rat endometriosis model | [94] |
| Baicalein | |||
| Cell cycle arrest, ↓Cell viability ↑Apoptosis | NF-κB inhibition, ↑Cells in the G1 phase ↓Cells in the S and G2/M phases, ↓BCL-2 | Endometrial stromal cells from patients with ovarian endometriosis | [95] |
| ↓Cell invasiveness | ↓MMP-9, ↓MMP-2, ↓MT1-MMP, ↓TGFB1, ↓FURIN | Ectopic endometrial stromal cells | [96] |
| ↓Cell invasiveness ↓Endometriotic lesion growth | ↓MT1-MMP, ↓FURIN, ↓TGFB1 | Murine endometriosis model | [96] |
| Wogonin | |||
| ↓Cell proliferation Cell cycle arrest | ↑Cells in the G2/M phase, ↓ERα | Endometrial stromal T-HESC cells Primary endometriotic stromal cells | [97] |
| ↓Endometriotic implant size ↓Cell proliferation, ↑Apoptosis | ↓Proliferating cells ↑Apoptotic cells | Murine endometriosis model | [97] |
| Rosmarinic acid | |||
| ↓Cell proliferation, ↓Oxidation | Cell cycle arrest in the G2/M phase ↓ROS | Endometrial stromal T-HESC cell line Primary endometriotic stromal cells | [97] |
| ↓Endometriotic tissue volume | ↓PCNA positive cells, ↑Apoptotic cells in lesions | Murine endometriosis model | [97] |
| Genistein and daidzein | |||
| ↓Cell proliferation ↓Inflammation ↓Estrogen biosynthesis | NF-κB inhibition, ↓IL6, ↓IL8, ↓COX2, ↓PGE2 Higher affinity toward ERβ than ERα ↓CYP19A1 ↓Aromatase activity ↓Glucocorticoid-regulated kinase in serum | Primary endometriotic stromal cells from ovarian endometrioma | [98] |
| ↓Endometriotic lesions growth | ↓Ki-67-positive cells | Murine endometriosis model | [98] |
| Genistein | |||
| ↓Endometriotic implant size | Antagonistic estrogen activity | Rat endometriosis model | [99] |
| Puerarin | |||
| ↓Cell invasion ↓Angiogenesis | ↓MMP-9 ↑TIMP-1 ↓ICAM-1 ↓VEGF | Primary endometriotic stromal cells Chick chorioallantoic membrane model | [100] |
| ↓Cell proliferation ↑Antiestrogen activity | ↓CCND1, ↓Cyclin D1, ↓CDC25A, ↓Cdc25A ↑ERα corepressors (SMRT, NCoR) ↓ERα coactivators (SRC-1, SRC-3) | Endometriotic stromal cells | [101] |
| ↑Antiestrogen activity | ↓CYP19A1, ↓aromatase activity, ↓HSD17B1, ↑HSD17B2, ↓COX-2, ↓COX2, ↓PGE2, ↓ERβ, ↓E2 | Rat endometriotic model | [102] |
| Naringenin | |||
| ↓Cell proliferation ↑Apoptosis | PI3K and MAPK pathway activation ↓Mitochondrial membrane potential, ↓ROS | Endometriosis cell lines | [103] |
| ↓Endometriotic lesions growth ↓Angiogenesis, ↓Inflammation ↑Apoptosis ↓Endometriosis prognostic markers | ↓MMP-2, ↓MMP-9, ↓TNF-α, ↓NO, ↑ROS ↓Mitochondrial membrane potential, ↓VEGF ↓BCL-2, ↓PCNA, ↑Caspase-3, ↑Cyt-C ↓Nrf2/HO1/NQO1 signaling, ↓TAK1, ↓PAK1 | Rat endometriosis model | [104] |
| Xanthohumol | |||
| ↓Endometriotic lesions growth ↓Angiogenesis | ↓PI3K ↓Microvessel density | Murine endometriosis model | [105] |
| Epigallocatechin gallate | |||
| ↓Cell proliferation, ↓Cell migration ↓Cell invasion, ↓Fibrogenesis | ↓MAPK signaling, ↓Smad signaling ↓αSMA, ↓Col-I, ↓CTGF, ↓FN | Endometriotic and endometrial stromal cells from patients | [106] |
| ↓Endometriotic implant size ↓Angiogenesis | ↓Microvessel number and size ↓VEGFC/VEGFR2 expression and signalling | Murine endometriosis model | [107] |
| ↓Endometriotic lesions growth ↑Apoptosis, ↓Angiogenesis | ↓Lesion size and weight, ↓Serum VEGF ↑Apoptotic cells in lesions, ↓αSMA, ↓CD31 | Murine endometriosis model | [108] |
| Curcumin | |||
| ↑Cell adhesion ↓Inflammation | ↓ICAM1, ↓ICAM-1, ↓VCAM1, ↓VCAM-1 ↓IL-6, ↓IL-8, ↓MCP-1, NF-κB inhibition | Primary endometriotic stromal cells | [109] |
| ↓Cell proliferation | ↓E2 production | Primary endometriotic stromal cells | [110] |
| Cell cycle arrest ↑Apoptosis | ↑Cells in the G1 phase, ↓Cells in the S phase ↓VEGF | Primary endometriotic and endometrial stromal cells | [111] |
| ↓Inflammation ↓Angiogenesis | ↓IL-6, ↓IL-8, ↓IP-10, ↓G-CSF, ↓MCP-1 ↓RANTES | Primary endometriotic stromal cells derived from eutopic endometrium | [112] |
| ↑Apoptosis ↓Endometriotic lesions growth | Cytochrome c-mediated mitochondrial pathway modulation, p53-dependent and -independent pathway modulation, NF-κB inhibition, ↓MMP-3 | Murine endometriosis model | [113] |
| ↓Inflammation, ↓Oxidation | ↓MMP-9, ↓TNF-α, ↓Lipid and protein oxidation | Murine endometriosis model | [114] |
| ↓Endometriotic tissues weight and volume | ↓VEGF | Rat endometriosis model | [115] |
| ↓Angiogenesis ↓Endometriotic lesions growth | ↓Microvessel density | Rat endometriosis model | [116] |
| Resveratrol | |||
| ↓Cell invasion | ↓Cell invasion in Matrigel | Primary endometriotic stromal cells | [117] |
| Estrogenic activity | Dose-dependent agonistic and antagonistic activity | Endometrial Ishikawa cell line | [118] |
| ↓Cell proliferation, ↑Apoptosis | ↓Cell number, DNA fragmentation | Primary endometrial epithelial cells | [119] |
| ↓Endometriotic lesion number and volume, ↓Cell proliferation ↑Apoptosis, ↓Angiogenesis | ↓PCNA, ↓CD34, ↓Peritoneal VEGF ↓Vascular density area, DNA fragmentation | Murine endometriosis model | [119] |
| ↓Cell proliferation, ↑Apoptosis | ↓MKI67, ↑PCNA, DNA fragmentation | Murine endometriosis model | [117] |
| ↓Cell proliferation | ↓ERα, ↓Ki-67 | Immunocompromised mouse endometriosis model (RAG-2 knockout) | [118] |
| ↓Cell proliferation | ↓IGF-1, ↓HGF, ↓HGF | Primary eutopic and ectopic endometrial stromal cells from endometriosis patients | [120] |
| ↓Cell proliferation, ↑Apoptosis ↓Angiogenesis | ↑P53, ↑BAX, ↑CASP3, ↑SIRT1, ↓NO | 3D primary endometriotic and endometrial cell cultures | [121] |
| ↓Inflammation | ↓MCP1, ↓IL6, ↓IL8, ↓MCP-1, ↓IL-6, ↓IL-8, ↓RANTES | Primary ectopic endometrial stromal cells | [122] |
| ↓Endometriotic lesion size ↓Angiogenesis, ↓Inflammation | ↓VEGF in peritoneal fluid, plasma and tissue ↓MCP-1 in peritoneal fluid and plasma | Rat endometriosis model | [123] |
| ↓Endometriotic lesion size ↓Angiogenesis, ↓Inflammation | ↓VEGF in peritoneal fluid and endometriotic tissue. ↓MCP-1 in peritoneal fluid | Rat endometriosis model | [124] |
| ↓Endometriotic lesion size ↓Oxidation | ↑SOD activity in serum and tissue ↑GPX activity in serum and tissue | Rat endometriosis model | [125] |
| ↓Cell proliferation, ↓Cell migration ↓Cell invasion | EMT process suppression ↓MTA1, ↓ZEB2 | Primary endometrial stromal cells Murine endometriosis model | [126] |
| ↓Endometriosis-related pain | ↓aromatase activity, ↓COX-2 | Patients treated for 2 months with 30 mg resveratrol to the hormone therapy | [127] |
| ↓Cell invasion | ↓MMP2, ↓MMP9, ↓MMP-2, ↓MMP-9 in endometrial tissue, fluid and serum | 34 patients with endometriotic infertility treated with 400 mg resveratrol twice daily for 12–14 weeks with contraceptives | [128] |
| ↓ Angiogenesis, ↓Inflammation | ↓VEGF, ↓TNF, ↓VEGF, ↓TNF-α in the eutopic endometrial tissue | 34 patients with endometriosis within the implantation window treated with 400 mg resveratrol for 12–14 weeks | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołąbek, A.; Kowalska, K.; Olejnik, A. Polyphenols as a Diet Therapy Concept for Endometriosis—Current Opinion and Future Perspectives. Nutrients 2021, 13, 1347. https://doi.org/10.3390/nu13041347
Gołąbek A, Kowalska K, Olejnik A. Polyphenols as a Diet Therapy Concept for Endometriosis—Current Opinion and Future Perspectives. Nutrients. 2021; 13(4):1347. https://doi.org/10.3390/nu13041347
Chicago/Turabian StyleGołąbek, Agata, Katarzyna Kowalska, and Anna Olejnik. 2021. "Polyphenols as a Diet Therapy Concept for Endometriosis—Current Opinion and Future Perspectives" Nutrients 13, no. 4: 1347. https://doi.org/10.3390/nu13041347
APA StyleGołąbek, A., Kowalska, K., & Olejnik, A. (2021). Polyphenols as a Diet Therapy Concept for Endometriosis—Current Opinion and Future Perspectives. Nutrients, 13(4), 1347. https://doi.org/10.3390/nu13041347