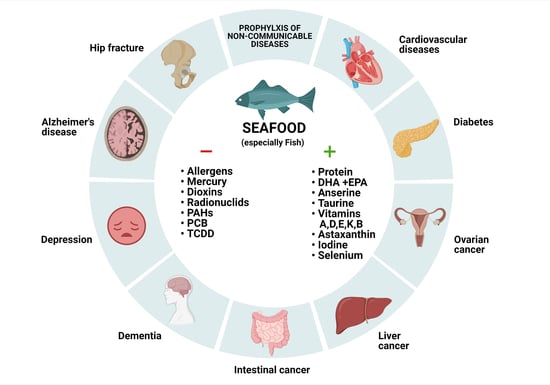
Seafood Intake as a Method of Non-Communicable Diseases (NCD) Prevention in Adults
Abstract
:1. Introduction
2. Nutrients and Bioactive Compound
2.1. Eicosapentaenoic Acid and Docosahexaenoic Acid
2.2. Astaxanthin and Tocopherols
2.3. Protein
2.4. Taurine and Anserine
3. Potential Health Benefits
3.1. Cardiovascular Diseases and Mortality
3.2. Metabolic Syndrome and T2DM
3.3. Cancer
3.4. Cognitive Impairment
4. Culinary Determinants of Seafood Health Properties
5. Seafood Safety
5.1. Pollution in Seafood
5.2. Seafood Allergy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Khan, F.; Orson, F.; Ogawa, Y.; Parker, C.; Davis, C.M. Adult Seafood Allergy in the Texas Medical Center: A 13-Year Experience. Allergy Rhinol. 2011, 2, e71–e77. [Google Scholar] [CrossRef] [PubMed]
- Nesheim, M.C.; Oria, M.; Yih, P.T.; National Research Council; Institute of Medicine; Food and Nutrition Board; Board on Agriculture and Natural Resources; Committee on a Framework for Assessing the Health, Environmental, and Social Effects of the Food System. Dietary Recommendations for Fish Consumption; The National Academies Press (USA): Washington, DC, USA, 2015. [Google Scholar] [CrossRef] [Green Version]
- Center for Food Safety and Applied Nutrition. Advice about Eating Fish. 2020. Available online: https://www.fda.gov/food/consumers/advice-about-eating-fish (accessed on 18 March 2021).
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Aickin, M.; Champagne, C.; Craddick, S.; Sacks, F.M.; McCarron, P.; Most-Windhauser, M.M.; Rukenbrod, F.; Haworth, L.; Dash-Sodium Collaborative Research Group. Food Group Sources of Nutrients in the Dietary Patterns of the DASH-Sodium Trial. J. Am. Diet. Assoc. 2003, 103, 488–496. [Google Scholar] [CrossRef]
- Zeng, L.; Ruan, M.; Liu, J.; Wilde, P.; Naumova, E.N.; Mozaffarian, D.; Zhang, F.F. Trends in Processed Meat, Unprocessed Red Meat, Poultry, and Fish Consumption in the United States, 1999–2016. J. Acad. Nutr. Diet. 2019, 119, 1085–1098. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L. U.S. Adults Are Not Meeting Recommended Levels for Fish and Omega-3 Fatty Acid Intake: Results of an Analysis Using Observational Data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Dashti, H.S.; Wojczynski, M.K.; Chu, A.Y.; Nettleton, J.A.; Männistö, S.; Kristiansson, K.; Reedik, M.; Lahti, J.; Houston, D.K.; et al. Genome-Wide Association Meta-Analysis of Fish and EPA+DHA Consumption in 17 US and European Cohorts. PLoS ONE 2017, 12, e0186456. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Morris, M.-C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.-F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Noncommunicable Diseases Progress Monitor 2020. Available online: https://www.who.int/publications/i/item/ncd-progress-monitor-2020 (accessed on 17 March 2021).
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Sun, N.; Ding, J.; Zhu, B.; Lin, S. Evaluation and Structure-Activity Relationship Analysis of Antioxidant Shrimp Peptides. Food Funct. 2019, 10, 5605–5615. [Google Scholar] [CrossRef] [PubMed]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function. Mol. Nutr. Food Res. 2019, 63, e1800569. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolińska, W.; Grela, E.R.; Bik-Małodzińska, M. Nutrient and Mineral Profile of Chosen Fresh and Smoked Fish. Nutrients 2019, 11, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daviglus, M.L.; Stamler, J.; Orencia, A.J.; Dyer, A.R.; Liu, K.; Greenland, P.; Walsh, M.K.; Morris, D.; Shekelle, R.B. Fish Consumption and the 30-Year Risk of Fatal Myocardial Infarction. N. Engl. J. Med. 1997, 336, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Hennekens, C.H.; O’Donnell, C.J.; Ajani, U.A.; Carey, V.J.; Willett, W.C.; Ruskin, J.N.; Manson, J.E. Fish Consumption and Risk of Sudden Cardiac Death. JAMA 1998, 279, 23–28. [Google Scholar] [CrossRef]
- Yuan, J.M.; Ross, R.K.; Gao, Y.T.; Yu, M.C. Fish and Shellfish Consumption in Relation to Death from Myocardial Infarction among Men in Shanghai, China. Am. J. Epidemiol. 2001, 154, 809–816. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Yan, W.; Zhu, J.; Zhu, Y.; Chen, J. Age-Related Disease Burden in China, 1997–2017: Findings from the Global Burden of Disease Study. Front. Public Health 2021, 9. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Hamid, F.; Pati, S.; Atun, R.; Millett, C. Impact of Noncommunicable Disease Multimorbidity on Healthcare Utilisation and Out-Of-Pocket Expenditures in Middle-Income Countries: Cross Sectional Analysis. PLoS ONE 2015, 10, e0127199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New Evidence Pyramid. BMJ Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [Green Version]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Afonso, C.; Bandarra, N.M.; Nunes, L.; Cardoso, C. Tocopherols in Seafood and Aquaculture Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 128–140. [Google Scholar] [CrossRef]
- Watanabe, F.; Bito, T. Vitamin B12 Sources and Microbial Interaction. Exp. Biol. Med. (Maywood) 2018, 243, 148–158. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (Vitamin B-2) and Health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- de Mello, V.D.F.; Schwab, U.; Kolehmainen, M.; Koenig, W.; Siloaho, M.; Poutanen, K.; Mykkänen, H.; Uusitupa, M. A Diet High in Fatty Fish, Bilberries and Wholegrain Products Improves Markers of Endothelial Function and Inflammation in Individuals with Impaired Glucose Metabolism in a Randomised Controlled Trial: The Sysdimet Study. Diabetologia 2011, 54, 2755–2767. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Mesa, M.D.; Gil, A. Omega-3 Long-Chain Polyunsaturated Fatty Acids Supplementation on Inflammatory Biomakers: A Systematic Review of Randomised Clinical Trials. Br. J. Nutr. 2012, 107 (Suppl. 2), S159–S170. [Google Scholar] [CrossRef] [Green Version]
- Robinson, L.E.; Mazurak, V.C. N-3 Polyunsaturated Fatty Acids: Relationship to Inflammation in Healthy Adults and Adults Exhibiting Features of Metabolic Syndrome. Lipids 2013, 48, 319–332. [Google Scholar] [CrossRef] [PubMed]
- von Schacky, C. N-3 Fatty Acids and the Prevention of Coronary Atherosclerosis. Am. J. Clin. Nutr. 2000, 71, 224S–227S. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids and Cardiovascular Disease: The Epidemiological Evidence. Environ. Health Prev. Med. 2002, 6, 203–209. [Google Scholar] [CrossRef]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription Omega-3 Fatty Acids and Their Lipid Effects: Physiologic Mechanisms of Action and Clinical Implications. Expert Rev. Cardiovasc. 2008, 6, 391–409. [Google Scholar] [CrossRef]
- Abeywardena, M.Y.; Patten, G.S. Role of Ω3 Long-Chain Polyunsaturated Fatty Acids in Reducing Cardio-Metabolic Risk Factors. Endocr. Metab. Immune. Disord. Drug Targets 2011, 11, 232–246. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the Global Burden of Alzheimer’s Disease. Alzheimers Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Solovyev, N.D. Importance of Selenium and Selenoprotein for Brain Function: From Antioxidant Protection to Neuronal Signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Wrzosek, M.; Łukaszkiewicz, J.; Wrzosek, M.; Jakubczyk, A.; Matsumoto, H.; Piątkiewicz, P.; Radziwoń-Zaleska, M.; Wojnar, M.; Nowicka, G. Vitamin D and the Central Nervous System. Pharmacol. Rep. 2013, 65, 271–278. [Google Scholar] [CrossRef]
- Rimmelzwaan, L.M.; van Schoor, N.M.; Lips, P.; Berendse, H.W.; Eekhoff, E.M.W. Systematic Review of the Relationship between Vitamin D and Parkinson’s Disease. J. Parkinsons Dis. 2016, 6, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C.; et al. ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M.; Christie, W.W. Seasonal Changes in Lipid Composition of Sardine (Sardina Pilchardus). J. Food Sci. 1997, 62, 40–42. [Google Scholar] [CrossRef]
- Jensen, K.N.; Jacobsen, C.; Nielsen, H.H. Fatty Acid Composition of Herring (Clupea Harengus L.): Influence of Time and Place of Catch on n-3 PUFA Content. J. Sci. Food AGR 2007, 87, 710–718. [Google Scholar] [CrossRef]
- Zheng, X.; Leaver, M.J.; Tocher, D.R. Long-Chain Polyunsaturated Fatty Acid Synthesis in Fish: Comparative Analysis of Atlantic Salmon (Salmo Salar L.) and Atlantic Cod (Gadus Morhua L.) Delta6 Fatty Acyl Desaturase Gene Promoters. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 255–263. [Google Scholar] [CrossRef]
- Jensen, I.J.; Mæhre, H.K.; Tømmerås, S.; Eilertsen, K.E.; Olsen, R.L.; Elvevoll, E.O. Farmed Atlantic Salmon (Salmo Salar L.) Is a Good Source of Long Chain Omega-3 Fatty Acids. Nutr. Bull. 2012, 37, 25–29. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of Sustainable Feeds on Omega-3 Long-Chain Fatty Acid Levels in Farmed Atlantic Salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef] [Green Version]
- Balzano, M.; Pacetti, D.; Lucci, P.; Fiorini, D.; Frega, N.G. Bioactive Fatty Acids in Mantis Shrimp, Crab and Caramote Prawn: Their Content and Distribution among the Main Lipid Classes. J. Food Compos. Anal. 2017, 59, 88–94. [Google Scholar] [CrossRef]
- Méndez, E.; González, R.M. Seasonal Changes in the Chemical and Lipid Composition of Fillets of the Southwest Atlantic Hake (Merluccius Hubbsi). Food Chem. 1997, 59, 213–217. [Google Scholar] [CrossRef]
- Ågren, J.; Muje, P.; Hänninen, O.; Herranen, J.; Penttilä, I. Seasonal Variations of Lipid Fatty Acids of Boreal Freshwater Fish Species. Comp. Biochem. Physiol. Part. B Comp. Biochem. 1987, 88, 905–909. [Google Scholar] [CrossRef]
- Guler, G.O.; Kiztanir, B.; Aktumsek, A.; Citil, O.B.; Ozparlak, H. Determination of the Seasonal Changes on Total Fatty Acid Composition and ω3/ω6 Ratios of Carp (Cyprinus Carpio L.) Muscle Lipids in Beysehir Lake (Turkey). Food Chem. 2008, 108, 689–694. [Google Scholar] [CrossRef]
- Kołakowska, A.; Domiszewski, Z.; Bienkiewicz, G. Effects of biological and technological factors on the utility of fish as a source of n-3 PUFA. In Omega 3 Fatty Acid Research; Nova Science Publishers: Hauppauge, NY, USA, 2006; pp. 83–107. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA) and Docosapentaenoic Acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar] [CrossRef]
- Miyawaki, H.; Takahashi, J.; Tsukahara, H.; Takehara, I. Effects of Astaxanthin on Human Blood Rheology. J. Clin. Biochem. Nutr. 2008, 43, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Turujman, S.A.; Wamer, W.G.; Wei, R.R.; Albert, R.H. Rapid Liquid Chromatographic Method to Distinguish Wild Salmon from Aquacultured Salmon Fed Synthetic Astaxanthin. J. AOAC Int. 1997, 80, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Spiller, G.A.; Dewell, A. Safety of an Astaxanthin-Rich Haematococcus Pluvialis Algal Extract: A Randomized Clinical Trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-Inhibitory Effects of the Astaxanthin-Rich Alga Haematococcus Pluvialis in Human Colon Cancer Cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Panel on Dietary Antioxidants and Related Compounds. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000; ISBN 978-0-309-06949-6. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci 2008, 4, 89–96. [Google Scholar]
- Wu, G. Functional Amino Acids in Growth, Reproduction, and Health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Paul, B.; Sarma, D.; Mathew, S.; Asha, K.K.; et al. Amino Acid Compositions of 27 Food Fishes and Their Importance in Clinical Nutrition. J. Amino Acids 2014, 2014, e269797. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Marois, J.; Weisnagel, S.J.; Jacques, H. Dietary Cod Protein Improves Insulin Sensitivity in Insulin-Resistant Men and Women: A Randomized Controlled Trial. Diabetes Care 2007, 30, 2816–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacaille, B.; Julien, P.; Deshaies, Y.; Lavigne, C.; Brun, L.D.; Jacques, H. Responses of Plasma Lipoproteins and Sex Hormones to the Consumption of Lean Fish Incorporated in a Prudent-Type Diet in Normolipidemic Men. J. Am. Coll. Nutr. 2000, 19, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Beauchesne-Rondeau, E.; Gascon, A.; Bergeron, J.; Jacques, H. Plasma Lipids and Lipoproteins in Hypercholesterolemic Men Fed a Lipid-Lowering Diet Containing Lean Beef, Lean Fish, or Poultry. Am. J. Clin. Nutr. 2003, 77, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, H.M.; Montero, M.P.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Mørkøre, T.; Borderías, J. Collagen Characteristics of Farmed Atlantic Salmon with Firm and Soft Fillet Texture. Food Chem. 2012, 134, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2019, 87, 187–254. [Google Scholar] [CrossRef]
- Laidlaw, S.A.; Grosvenor, M.; Kopple, J.D. The Taurine Content of Common Foodstuffs. JPEN J. Parenter Enter. Nutr. 1990, 14, 183–188. [Google Scholar] [CrossRef]
- Spitze, A.R.; Wong, D.L.; Rogers, Q.R.; Fascetti, A.J. Taurine Concentrations in Animal Feed Ingredients; Cooking Influences Taurine Content. J. Anim. Physiol. Anim. Nutr. 2003, 87, 251–262. [Google Scholar] [CrossRef]
- Bertinaria, M.; Rolando, B.; Giorgis, M.; Montanaro, G.; Guglielmo, S.; Buonsanti, M.F.; Carabelli, V.; Gavello, D.; Daniele, P.G.; Fruttero, R.; et al. Synthesis, Physicochemical Characterization, and Biological Activities of New Carnosine Derivatives Stable in Human Serum as Potential Neuroprotective Agents. J. Med. Chem. 2011, 54, 611–621. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Mannion, A.F.; Jakeman, P.M.; Dunnett, M.; Harris, R.C.; Willan, P.L. Carnosine and Anserine Concentrations in the Quadriceps Femoris Muscle of Healthy Humans. Eur. J. Appl. Physiol. Occup Physiol. 1992, 64, 47–50. [Google Scholar] [CrossRef]
- Everaert, I.; Baron, G.; Barbaresi, S.; Gilardoni, E.; Coppa, C.; Carini, M.; Vistoli, G.; Bex, T.; Stautemas, J.; Blancquaert, L.; et al. Development and Validation of a Sensitive LC-MS/MS Assay for the Quantification of Anserine in Human Plasma and Urine and Its Application to Pharmacokinetic Study. Amino Acids 2019, 51, 103–114. [Google Scholar] [CrossRef]
- Saour, B.; Smith, B.; Yancy, C.W. Heart Failure and Sudden Cardiac Death. Card. Electrophysiol. Clin. 2017, 9, 709–723. [Google Scholar] [CrossRef]
- Meijers, W.C.; de Boer, R.A. Common Risk Factors for Heart Failure and Cancer. Cardiovasc. Res. 2019, 115, 844–853. [Google Scholar] [CrossRef]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.-T.; Tsuji, I.; et al. Meat Intake and Cause-Specific Mortality: A Pooled Analysis of Asian Prospective Cohort Studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.P.; Thomas, G.N.; Ho, S.Y.; Lai, H.K.; Mak, K.H.; Lam, T.H. Fish Consumption and Mortality in Hong Kong Chinese--the LIMOR Study. Ann. Epidemiol. 2011, 21, 164–169. [Google Scholar] [CrossRef]
- Osler, M.; Andreasen, A.H.; Hoidrup, S. No Inverse Association between Fish Consumption and Risk of Death from All-Causes, and Incidence of Coronary Heart Disease in Middle-Aged, Danish Adults. J. Clin. Epidemiol. 2003, 56, 274–279. [Google Scholar] [CrossRef]
- Gillum, R.F.; Mussolino, M.; Madans, J.H. The Relation between Fish Consumption, Death from All Causes, and Incidence of Coronary Heart Disease. the NHANES I Epidemiologic Follow-up Study. J. Clin. Epidemiol. 2000, 53, 237–244. [Google Scholar] [CrossRef]
- Olsen, A.; Egeberg, R.; Halkjær, J.; Christensen, J.; Overvad, K.; Tjønneland, A. Healthy Aspects of the Nordic Diet Are Related to Lower Total Mortality. J. Nutr. 2011, 141, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake with Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef]
- Jayedi, A.; Shab-Bidar, S.; Eimeri, S.; Djafarian, K. Fish Consumption and Risk of All-Cause and Cardiovascular Mortality: A Dose–Response Meta-Analysis of Prospective Observational Studies. Public Health Nutr. 2018, 21, 1297–1306. [Google Scholar] [CrossRef]
- Djoussé, L.; Akinkuolie, A.O.; Wu, J.H.Y.; Ding, E.L.; Gaziano, J.M. Fish Consumption, Omega-3 Fatty Acids and Risk of Heart Failure: A Meta-Analysis. Clin. Nutr. 2012, 31, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.-G.; Sun, J.-W.; Yang, Y.; Ma, X.; Wang, Y.-Y.; Xiang, Y.-B. Fish Consumption and All-Cause Mortality: A Meta-Analysis of Cohort Studies. Eur. J. Clin. Nutr. 2016, 70, 155–161. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the Metabolic Syndrome among US Adults: Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef]
- Mozumdar, A.; Liguori, G. Persistent Increase of Prevalence of Metabolic Syndrome among U.S. Adults: NHANES III to NHANES 1999–2006. Diabetes Care 2011, 34, 216–219. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-S.; Xun, P.; He, K. Fish Consumption, Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Metabolic Syndrome: A Meta-Analysis. Nutrients 2015, 7, 2085–2100. [Google Scholar] [CrossRef] [Green Version]
- Muley, A.; Muley, P.; Shah, M. ALA, Fatty Fish or Marine n-3 Fatty Acids for Preventing DM?: A Systematic Review and Meta-Analysis. Curr. Diabetes Rev. 2014, 10, 158–165. [Google Scholar] [CrossRef]
- Kolahdooz, F.; van der Pols, J.C.; Bain, C.J.; Marks, G.C.; Hughes, M.C.; Whiteman, D.C.; Webb, P.M.; Australian Cancer Study (Ovarian Cancer) and the Australian Ovarian Cancer Study Group. Meat, Fish, and Ovarian Cancer Risk: Results from 2 Australian Case-Control Studies, a Systematic Review, and Meta-Analysis. Am. J. Clin. Nutr. 2010, 91, 1752–1763. [Google Scholar] [CrossRef] [Green Version]
- Tavani, A.; Pelucchi, C.; Parpinel, M.; Negri, E.; Franceschi, S.; Levi, F.; La Vecchia, C. N-3 Polyunsaturated Fatty Acid Intake and Cancer Risk in Italy and Switzerland. Int. J. Cancer 2003, 105, 113–116. [Google Scholar] [CrossRef]
- Bandera, E.V.; Kushi, L.H.; Moore, D.F.; Gifkins, D.M.; McCullough, M.L. Consumption of Animal Foods and Endometrial Cancer Risk: A Systematic Literature Review and Meta-Analysis. Cancer Causes Control 2007, 18, 967–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zeng, R.; Huang, J.; Li, X.; Zhang, J.; Ho, J.C.-M.; Zheng, Y. Dietary Protein Sources and Incidence of Breast Cancer: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients 2016, 8, 730. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.-S.; Hu, X.-J.; Zhao, Y.-M.; Yang, J.; Li, D. Intake of Fish and Marine N-3 Polyunsaturated Fatty Acids and Risk of Breast Cancer: Meta-Analysis of Data from 21 Independent Prospective Cohort Studies. BMJ (Clin. Res. Ed.) 2013, 346, f3706. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Spiegelman, D.; Hou, T.; Albanes, D.; Allen, N.E.; Berndt, S.I.; van den Brandt, P.A.; Giles, G.G.; Giovannucci, E.; Alexandra Goldbohm, R.; et al. Associations between Unprocessed Red and Processed Meat, Poultry, Seafood and Egg Intake and the Risk of Prostate Cancer: A Pooled Analysis of 15 Prospective Cohort Studies. Int. J. Cancer 2016, 138, 2368–2382. [Google Scholar] [CrossRef]
- Szymanski, K.M.; Wheeler, D.C.; Mucci, L.A. Fish Consumption and Prostate Cancer Risk: A Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1223–1233. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and Beverages and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies, an Update of the Evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of Fish and Polyunsaturated Fatty Acids and Mild-to-Severe Cognitive Impairment Risks: A Dose-Response Meta-Analysis of 21 Cohort Studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef]
- Jayedi, A.; Shab-Bidar, S. Fish Consumption and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Adv. Nutr. 2020, 11, 1123–1133. [Google Scholar] [CrossRef]
- Joshi, A.D.; John, E.M.; Koo, J.; Ingles, S.A.; Stern, M.C. Fish Intake, Cooking Practices, and Risk of Prostate Cancer: Results from a Multi-Ethnic Case-Control Study. Cancer Causes Control 2012, 23, 405–420. [Google Scholar] [CrossRef]
- Cai, L.; Zheng, Z.-L.; Zhang, Z.-F. Risk Factors for the Gastric Cardia Cancer: A Case-Control Study in Fujian Province. World J. Gastroenterol. 2003, 9, 214–218. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.P.; Woodrow, J.; Zhu, Y.; Roebothan, B.; Mclaughlin, J.R.; Parfrey, P.S. Dietary Patterns and Colorectal Cancer: Results from a Canadian Population-Based Study. Nutr. J. 2015, 14, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Lemaitre, R.N.; Kuller, L.H.; Burke, G.L.; Tracy, R.P.; Siscovick, D.S. Cardiovascular Health Study Cardiac Benefits of Fish Consumption May Depend on the Type of Fish Meal Consumed: The Cardiovascular Health Study. Circulation 2003, 107, 1372–1377. [Google Scholar] [CrossRef]
- Owen, A.J.; Magliano, D.J.; O’Dea, K.; Barr, E.L.M.; Shaw, J.E. Polyunsaturated Fatty Acid Intake and Risk of Cardiovascular Mortality in a Low Fish-Consuming Population: A Prospective Cohort Analysis. Eur. J. Nutr. 2016, 55, 1605–1613. [Google Scholar] [CrossRef]
- Takata, Y.; Zhang, X.; Li, H.; Gao, Y.-T.; Yang, G.; Gao, J.; Cai, H.; Xiang, Y.-B.; Zheng, W.; Shu, X.-O. Fish Intake and Risks of Total and Cause-Specific Mortality in 2 Population-Based Cohort Studies of 134,296 Men and Women. Am. J. Epidemiol. 2013, 178, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-Q.; Terry, P.D.; Yan, H. Review of Salt Consumption and Stomach Cancer Risk: Epidemiological and Biological Evidence. World J. Gastroenterol. 2009, 15, 2204–2213. [Google Scholar] [CrossRef]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological Implications of Lipid Oxidation Products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of Fried Food Consumption with All Cause, Cardiovascular, and Cancer Mortality: Prospective Cohort Study. BMJ 2019, 364, k5420. [Google Scholar] [CrossRef] [Green Version]
- Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Lopez-Garcia, E.; León-Muñoz, L.M.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Buckland, G.; Chirlaque, M.-D.; et al. Consumption of Fried Foods and Risk of Coronary Heart Disease: Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition Study. BMJ 2012, 344, e363. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.S.; Galano, J.-M.; Durand, T.; Lee, J.C.-Y. Profiling of Omega-Polyunsaturated Fatty Acids and Their Oxidized Products in Salmon after Different Cooking Methods. Antioxidants 2018, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Domiszewski, Z.; Duszyńska, K.; Stachowska, E. Influence of Different Heat Treatments on the Lipid Quality of African Catfish (Clarias Gariepinus). J. Aquat. Food Prod. Technol. 2020, 29, 886–900. [Google Scholar] [CrossRef]
- Kendall, P.; Hillers, V.; Medeiros, L. Food Safety Guidance for Older Adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 42, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Karl, H.; Kammann, U.; Aust, M.-O.; Manthey-Karl, M.; Lüth, A.; Kanisch, G. Large Scale Distribution of Dioxins, PCBs, Heavy Metals, PAH-Metabolites and Radionuclides in Cod (Gadus Morhua) from the North Atlantic and Its Adjacent Seas. Chemosphere 2016, 149, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Struciński, P.; Piskorska-Pliszczynska, J.; Góralczyk, K.; Warenik-Bany, M.; Maszewski, S.; Czaja, K.; Ludwicki, J. Dioxins and Food Safety. Rocz. Państwowego Zakładu Hig. 2011, 62, 3–17. [Google Scholar]
- Leventakou, V.; Roumeliotaki, T.; Martinez, D.; Barros, H.; Brantsaeter, A.-L.; Casas, M.; Charles, M.-A.; Cordier, S.; Eggesbø, M.; van Eijsden, M.; et al. Fish Intake during Pregnancy, Fetal Growth, and Gestational Length in 19 European Birth Cohort Studies. Am. J. Clin. Nutr. 2014, 99, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assessment and Management of Seafood Safety and Quality. Available online: http://www.fao.org/3/y4743e/y4743e0e.htm (accessed on 18 March 2021).
- Gdula-Argasińska, J.; Czepiel, J.; Woźniakiewicz, A.; Wojtoń, K.; Grzywacz, A.; Woźniakiewicz, M.; Jurczyszyn, A.; Perucki, W.; Librowski, T. N-3 Fatty Acids as Resolvents of Inflammation in the A549 Cells. Pharm. Rep. 2015, 67, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Donat-Vargas, C.; Berglund, M.; Glynn, A.; Wolk, A.; Åkesson, A. Dietary Polychlorinated Biphenyls, Long-Chain n-3 Polyunsaturated Fatty Acids and Incidence of Malignant Melanoma. Eur. J. Cancer 2017, 72, 137–143. [Google Scholar] [CrossRef]
- Turkez, H.; Geyikoglu, F.; Yousef, M.I. Ameliorative Effects of Docosahexaenoic Acid on the Toxicity Induced by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin in Cultured Rat Hepatocytes. Toxicol. Ind. Health 2016, 32, 1074–1085. [Google Scholar] [CrossRef]
- Ireneusz, C.; Joanna, R.-T.; Monika, S.; Dobrzyński, M.; Andrzej, G. Zastosowanie Wysokich Dawek Tokoferolu w Prewencji i Potencjalizacji Działania Dioksyn w Doświadczalnym Zapaleniu. Postępy Hig. I Med. Doświadczalnej 2011, 65. [Google Scholar] [CrossRef]
- Nicklisch, S.C.T.; Bonito, L.T.; Sandin, S.; Hamdoun, A. Mercury Levels of Yellowfin Tuna (Thunnus Albacares) Are Associated with Capture Location. Environ. Pollut. 2017, 229, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Cammilleri, G.; Vazzana, M.; Arizza, V.; Giunta, F.; Vella, A.; Lo Dico, G.; Giaccone, V.; Giofrè, S.V.; Giangrosso, G.; Cicero, N.; et al. Mercury in Fish Products: What’s the Best for Consumers between Bluefin Tuna and Yellowfin Tuna? Nat. Prod. Res. 2018, 32, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Frantzen, S.; Måge, A.; Iversen, S.A.; Julshamn, K. Seasonal Variation in the Levels of Organohalogen Compounds in Herring (Clupea Harengus) from the Norwegian Sea. Chemosphere 2011, 85, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lundebye, A.-K.; Lock, E.-J.; Rasinger, J.D.; Nøstbakken, O.J.; Hannisdal, R.; Karlsbakk, E.; Wennevik, V.; Madhun, A.S.; Madsen, L.; Graff, I.E.; et al. Lower Levels of Persistent Organic Pollutants, Metals and the Marine Omega 3-Fatty Acid DHA in Farmed Compared to Wild Atlantic Salmon (Salmo Salar). Environ. Res. 2017, 155, 49–59. [Google Scholar] [CrossRef]
- Varol, M.; Sünbül, M.R. Comparison of Heavy Metal Levels of Farmed and Escaped Farmed Rainbow Trout and Health Risk Assessment Associated with Their Consumption. Environ. Sci. Pollut. Res. Int. 2017, 24, 23114–23124. [Google Scholar] [CrossRef] [PubMed]
- Hites, R.A.; Foran, J.A.; Carpenter, D.O.; Hamilton, M.C.; Knuth, B.A.; Schwager, S.J. Global Assessment of Organic Contaminants in Farmed Salmon. Science 2004, 303, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.D.; Brenner, D.; Berger, M.L.; Carpenter, D.O.; Hong, C.-S.; Kannan, K. PCBs, PCDD/Fs, and Organochlorine Pesticides in Farmed Atlantic Salmon from Maine, Eastern Canada, and Norway, and Wild Salmon from Alaska. Environ. Sci. Technol. 2006, 40, 5347–5354. [Google Scholar] [CrossRef] [PubMed]
- Nácher-Mestre, J.; Serrano, R.; Benedito-Palos, L.; Navarro, J.; López, F.; Kaushik, S.; Pérez-Sánchez, J. Bioaccumulation of Polycyclic Aromatic Hydrocarbons in Gilthead Sea Bream (Sparus Aurata L.) Exposed to Long Term Feeding Trials with Different Experimental Diets. Arch. Environ. Contam. Toxicol. 2010, 59, 137–146. [Google Scholar] [CrossRef] [Green Version]
- van Leeuwen, S.P.J.; Swart, C.P.; van der Veen, I.; de Boer, J. Significant Improvements in the Analysis of Perfluorinated Compounds in Water and Fish: Results from an Interlaboratory Method Evaluation Study. J. Chromatogr. A 2009, 1216, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Bienkiewicz, G.; Domiszewski, Z.; Tokarczyk, G.; Plust, D. Distribution of Lipids and Oxidative Changes Therein in Particularized Parts of Rainbow Trout Fillets. Zywnosc. Nauka. Technol. Jakosc Food. Sci. Technol. Qual. 2013, 20. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risk for Animal and Human Health Related to the Presence of Dioxins and Dioxin-like PCBs in Feed and Food. EFSA J. 2018, 16, e05333. [Google Scholar] [CrossRef] [Green Version]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Assessment of a Decontamination Process for Dioxins and PCBs from Fish Meal by Replacement of Fish Oil. EFSA J. 2018, 16, e05174. [Google Scholar] [CrossRef] [Green Version]
- Berntssen, M.H.G.; Sanden, M.; Hove, H.; Lie, Ø. Modelling Scenarios on Feed-to-Fillet Transfer of Dioxins and Dioxin-like PCBs in Future Feeds to Farmed Atlantic Salmon (Salmo Salar). Chemosphere 2016, 163, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, T.A.; Peterson, S.; Lau, C.H.; Saltoun, C.A.; Gupta, R.S.; Bryce, P.J. Prevalence and Characteristics of Adult-Onset Food Allergy. J. Allergy Clin. Immunol. Pract. 2015, 3, 114–115.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; et al. The Prevalence of Food Allergy: A Meta-Analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Mullins, R.J.; Wainstein, B.K.; Barnes, E.H.; Liew, W.K.; Campbell, D.E. Increases in Anaphylaxis Fatalities in Australia from 1997 to 2013. Clin. Exp. Allergy 2016, 46, 1099–1110. [Google Scholar] [CrossRef]
- Schabelman, E.; Witting, M. The Relationship of Radiocontrast, Iodine, and Seafood Allergies: A Medical Myth Exposed. J. Emerg. Med. 2010, 39, 701–707. [Google Scholar] [CrossRef]
- Beaty, A.D.; Lieberman, P.L.; Slavin, R.G. Seafood Allergy and Radiocontrast Media: Are Physicians Propagating a Myth? Am. J. Med. 2008, 121, 158-e1. [Google Scholar] [CrossRef]
- Dewachter, P.; Kopac, P.; Laguna, J.J.; Mertes, P.M.; Sabato, V.; Volcheck, G.W.; Cooke, P.J. Anaesthetic Management of Patients with Pre-Existing Allergic Conditions: A Narrative Review. Br. J. Anaesth. 2019, 123, e65–e81. [Google Scholar] [CrossRef]
- Pradubpongsa, P.; Dhana, N.; Jongjarearnprasert, K.; Janpanich, S.; Thongngarm, T. Adverse Reactions to Iodinated Contrast Media: Prevalence, Risk Factors and Outcome-the Results of a 3-Year Period. Asian Pac. J. Allergy Immunol. 2013, 31, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Schlifke, A.; Geiderman, J.M. Seafood Allergy Is a Specific and Unique Contraindication to the Administration of Ionic Contrast Media. CJEM 2003, 5, 166–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| References | Studies (N) | Participants (n) | Outcome | RR (95% Cl)/SRR * |
|---|---|---|---|---|
| Jayedi et al. (2018) [79] | 14 | 911,348 | All-cause mortality | 0.97 (0.96–0.98) |
| Djousse et al. (2012) [80] | 5 | 170,131 | Heart failure | 0.95 (0.93–0.98) |
| Zhao et al. (2016) [81] | 12 | 672,389 | All-cause mortality | 0.94 (0.90–0.98) |
| Kim et al. (2015) [84] | 9 | 7860 | Metabolic syndrome | 0.71 (0.58–0.87) |
| Muley et al. (2014) [85] | 16 | 679,763 | T2DM | 0.89 (0.801–0.987) |
| Kolahdooz et al. (2010) [86] | 6 | 16,886 | Ovarian cancer | 0.84 (0.68–1.03) |
| Bandera et al. (2007) [88] | 5 | 10,543 | Endometrial cancer | 1.88 (1.20–2.98) |
| Wu et al. (2016) [89] | 13 | 758,359 | Breast cancer | 1.07 (0.94–1.21) |
| Zheng et al. (2013) [90] | 11 | 687,770 | Breast cancer | 1.03 (0.93–1.14) |
| Wu et al. (2016) [91] | 52,683 | Prostate cancer | 1.04 (0.98–1.09) | |
| Szymański et al. (2010) [92] | 49,661 | Prostate cancer | 0.37 (0.18–0.74) | |
| Vieira et al. (2017) [93] | 11 | 3944 | Colorectal cancer | 0.89 (0.80–0.99) |
| Zhang et al. (2016) [94] | 4 | 21,099 | Dementia | 0.95 (0.90–0.99) |
| 5 | 21,941 | Alzheimer’s disease | 0.93 (0.90–0.95) | |
| Jayedi et al. (2020) [95] | 38 | 153,998 | All-cause mortality | 0.92 * (0.87–0.97) |
| 8 | 11,720 | Cardiovascular mortality | 0.75 * (0.65–0.87) | |
| 22 | 16,732 | Coronary heart disease | 0.88 * (0.79–0.99) | |
| 11 | 8468 | Myocardial infarction | 0.75 * (0.65–0.93) | |
| 20 | 14,360 | Stroke | 0.86 * (0.75–0.99) | |
| 8 | 7945 | Heart failure | 0.80 * (0.67–0.95) | |
| 8 | 5732 | Depression | 0.88 * (0.79–0.98) | |
| 5 | 1572 | Liver cancer | 0.65 * (0.48–0.87) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamioł-Milc, D.; Biernawska, J.; Liput, M.; Stachowska, L.; Domiszewski, Z. Seafood Intake as a Method of Non-Communicable Diseases (NCD) Prevention in Adults. Nutrients 2021, 13, 1422. https://doi.org/10.3390/nu13051422
Jamioł-Milc D, Biernawska J, Liput M, Stachowska L, Domiszewski Z. Seafood Intake as a Method of Non-Communicable Diseases (NCD) Prevention in Adults. Nutrients. 2021; 13(5):1422. https://doi.org/10.3390/nu13051422
Chicago/Turabian StyleJamioł-Milc, Dominika, Jowita Biernawska, Magdalena Liput, Laura Stachowska, and Zdzisław Domiszewski. 2021. "Seafood Intake as a Method of Non-Communicable Diseases (NCD) Prevention in Adults" Nutrients 13, no. 5: 1422. https://doi.org/10.3390/nu13051422
APA StyleJamioł-Milc, D., Biernawska, J., Liput, M., Stachowska, L., & Domiszewski, Z. (2021). Seafood Intake as a Method of Non-Communicable Diseases (NCD) Prevention in Adults. Nutrients, 13(5), 1422. https://doi.org/10.3390/nu13051422