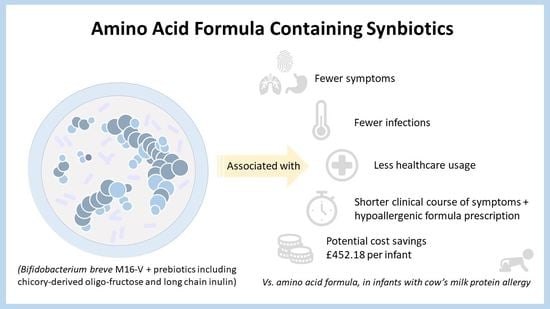
The Use of an Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy—Effect on Clinical Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. The Health Improvement Network (THIN) Database
2.3. Study Population
2.4. Study Variables and Outcome Measures
- Baseline (birth to diagnosis) characteristics. Due to limited detail within read-codes regarding symptom severity, this included several proxies for disease severity (presenting symptoms, number of organ systems affected, history of infections, other allergies, faltering growth, eHF prescription and the mean number of allergic medication prescriptions).
- Clinical symptoms. This included all-cause symptoms (symptoms documented in case records related to any cause) and allergic symptoms, including GI, skin and respiratory symptoms. Examples of symptoms classified as GI symptoms included GI illness, diarrhoea, constipation, flatulence, vomiting, reflux, bloody stools, mucus in stools and colic. Examples of skin symptoms included eczema and urticaria, and respiratory symptoms included asthma and rhinitis. GI, skin and respiratory symptoms were also aggregated into overall allergic symptoms.
- Infections. This included all-cause infections and infection sub-categories (GI, skin, respiratory and ear infections). GI, skin, respiratory and ear infections were also aggregated into overall allergic symptoms.
- Healthcare usage. This included all-cause prescriptions, prescriptions of antibiotics, dermatological medications, anti-reflux medications, all-cause healthcare contacts, Dietitian contacts, hospital admissions and specialist referrals. Prescriptions of antibiotics, dermatological medications and anti-reflux medications were aggregated into overall medication prescriptions, and Dietitian contacts, hospital admissions and specialist referrals were aggregated into overall healthcare contacts.
3. Results
3.1. Demographics
3.2. Management of CMPA
3.3. Disease Severity
3.4. Outcome Measures
3.4.1. All-Cause Symptoms, Infections and Healthcare Usage
3.4.2. Allergic Symptoms
3.4.3. GI, Skin, Respiratory and Ear Infections
3.4.4. Healthcare Usage
Antibiotic, Dermatological and Anti-Reflux Medications
Dietitian Contacts, Hospital Admissions and Specialist Referrals
3.4.5. Clinical Course of Symptoms and HAF Prescription
3.4.6. Indicative Cost-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ. J. 2010, 3, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Grimshaw, K.E.; Bryant, T.; Oliver, E.M.; Martin, J.; Maskell, J.; Kemp, T.; Clare Mills, E.N.; Foote, K.D.; Margetts, B.M.; Beyer, K.; et al. Incidence and risk factors for food hypersensitivity in UK infants: Results from a birth cohort study. Clin. Transl. Allergy 2015, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.; De Koker, C.; Dziubak, R.; Venter, C.; Dominguez-Ortega, G.; Cutts, R.; Yerlett, N.; Skrapak, A.K.; Fox, A.T.; Shah, N. Malnutrition in children with food allergies in the UK. J. Hum. Nutr. Diet. 2014, 27, 227–235. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnosis approach and management of cow’s milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Paediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Pract. 2017, 6, 383–399. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Clinical Knowledge Summaries: Cow’s Milk Allergy in Children. Available online: https://cks.nice.org.uk/topics/cows-milk-allergy-in-children/ (accessed on 7 April 2021).
- Sladkevicius, E.; Nagy, E.; Lack, G.; Guest, J.F. Resource implications and budget impact of managing cow milk allergy in the UK. J. Med. Econ. 2010, 13, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Firer, M.A.; Ball, G.; Hosking, C.S. Natural history of cows’ milk allergy in children: Immunological outcome over 2 years. Clin. Exp. Allergy 1993, 23, 124–131. [Google Scholar] [CrossRef]
- García-Ara, M.C.; Boyano-Martínez, M.T.; Díaz-Pena, J.M.; Martín-Muñoz, M.F.; Martín-Esteban, M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin. Exp. Allergy 2004, 34, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Terracciano, L.; Bouygue, G.R.; Veglia, F.; Sarratud, T.; Martelli, A.; Restani, P. Incremental prognostic factors associated with cow’s milk allergy outcomes in infant and child referrals: The Milan Cow’s Milk Allergy Cohort study. Ann. Allergy Asthma Immunol. 2008, 101, 166–173. [Google Scholar] [CrossRef]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Lopez Longo, M.N.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ho, H.E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 2019, 122, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef] [Green Version]
- Sierra, C.; Bernal, M.J.; Blasco, J.; Martínez, R.; Dalmau, J.; Ortuño, I.; Espín, B.; Vasallo, M.I.; Gil, D.; Vidal, M.L.; et al. Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: A multicentre, randomised, double-blind and placebo-controlled trial. Eur. J. Nutr. 2015, 54, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qamer, S.; Deshmukh, M.; Patole, S. Probiotics for cow’ milk protein allergy: A systematic review of randomized controlled trials. Eur. J. Pediatrics 2019, 178, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Veereman-Wauters, G.; Staelens, S.; Van de Broek, H.; Plaskie, K.; Wesling, F.; Roger, L.C.; McCartney, A.L.; Assam, P. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J. Pediatric Gastroenterol. Nutr. 2011, 52, 763–771. [Google Scholar] [CrossRef]
- Burks, A.W.; Harthoorn, L.F.; Van Ampting, M.T.; Nijhuis, M.M.O.; Langford, J.E.; Wopereis, H.; Goldberg, S.B.; Ong, P.Y.; Essink, B.J.; Scott, R.B.; et al. Synbiotics-supplemented amino acid-based formula supports adequate growth in cow’s milk allergic infants. Pediatric Allergy Immunol. 2015, 26, 316–322. [Google Scholar] [CrossRef]
- Candy, D.C.; Van Ampting, M.T.; Nijhuis, M.M.O.; Wopereis, H.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E.; et al. A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatric Res. 2018, 83, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.T.; Wopereis, H.; Van Ampting, M.T.J.; Nijhuis, M.M.O.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Candy, D.C.A.; Shah, N.; West, C.E.; et al. A specific synbiotic-containing amino acid-based formula in dietary management of cow’s milk allergy: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, K.; Cawood, A.L.; Gibson, G.R.; Cooke, L.H.; Stratton, R.J. Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.; Sangsupawanich, P.; Van Ampting, M.; Nijhuis, M.; Harthoorn, L.; Landgford, J.; et al. Tolerance Development in Infants with IgE Mediated Cow’s Milk Allergy Receiving. Amino Acid-Based Formula including Specific Synbiotics: A Multi-Center Randomized Controlled Clinical Trial (PRESTO). Presented at the EAACI PAAM, Florence, Italy, 17–19 October 2019. [Google Scholar]
- Blak, B.T.; Thompson, M.; Dattani, H.; Bourke, A. Generalisability of The Health Improvement Network (THIN) database: Demographics, chronic disease prevalence and mortality rates. Inform. Prim. Care 2011, 19, 251–255. [Google Scholar] [CrossRef]
- Benson, T. The history of the Read codes: The inaugural James Read Memorial Lecture 2011. J. Innov. Health Inform. 2011, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization Collaborating Centre for Drug Statistics Methodology. International Language for Drug Utilization Research—ATC/DDD. Available online: https://www.whocc.no/ (accessed on 11 January 2021).
- The Health Improvement Network THIN Research. Available online: https://www.the-health-improvement-network.com/en/#thin-research (accessed on 8 June 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 17 March 2021).
- Barraclough, H.; Simms, L.; Govindan, R. Biostatistics Primer: What a Clinician Ought to Know: Hazard Ratios. J. Thorac. Oncol. 2011, 6, 978–982. [Google Scholar] [CrossRef] [Green Version]
- National Information Center on Health Services Research and Health Care Technology. HTA 101: V. Economic Analysis Methods. Available online: https://www.nlm.nih.gov/nichsr/hta101/ta10107.html (accessed on 17 March 2021).
- Monthly Index of Medical Specialties. MIMS Online. Available online: https://www.mims.co.uk (accessed on 11 January 2021).
- Prescribing and Medicines Team NHS Digital. Prescription Cost Analysis: England 2018—2018 Data Tables. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed on 9 April 2021).
- Curtis, L.; Burns, A. Unit Costs of Health & Social Care. Available online: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/ (accessed on 9 April 2021).
- NHS Improvement and NHS England. 2020/21 National Tariff Payment System: National Prices and Prices for Blended Payments. Available online: https://improvement.nhs.uk/resources/national-tariff (accessed on 9 April 2021).
- Office for National Statistics. UK Population Pyramid Interactive. 2018. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/ukpopulationpyramidinteractive/2020-01-08 (accessed on 21 December 2020).
- Meyer, R.; Godwin, H.; Dziubak, R.; Panepinto, J.A.; Foong, R.-X.M.; Bryon, M.; Lozinsky, A.C.; Reeve, K.; Shah, N. The impact on quality of life on families of children on an elimination diet for Non-immunoglobulin E mediated gastrointestinal food allergies. World Allergy Organ. J. 2017, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Sun, L.; Wang, B.; Yang, X.; Shang, L.; Zhang, Y. Health-related quality of life among children with recurrent respiratory tract infections in Xi’an, China. PLoS ONE 2013, 8, e56945. [Google Scholar] [CrossRef]
- Meltzer, E.O. Quality of life in adults and children with allergic rhinitis. J. Allergy Clin. Immunol. 2001, 101, 45–53. [Google Scholar] [CrossRef]
- Wopereis, H.; Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.; Sangsupawanich, P.; de Weerd, H.; Kakourou, A.; Roeselers, G.; et al. Increased and Enriched Bifidobacterium Community in Gut Microbiota of Infants with IgE-Mediated CMA Receiving a Specific Synbiotic-Containing Amino Acid-Based Formula. Presented at the EAACI FAAM-EUROBAT, Digital Event, 16–17 October 2020. [Google Scholar]
- Wopereis, H.; van Ampting, M.T.J.; Cetinyurek-Yavuz, A.; Slump, R.; Candy, D.C.A.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; et al. A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow’s milk allergic infants: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 27. [Google Scholar] [CrossRef]
- Agostoni, C.; Axelsson, I.; Braegger, C.; Goulet, O.; Koletzko, B.; Michaelsen, K.F.; Rigo, J.; Shamir, R.; Szajewska, H.; Turck, D. Probiotic bacteria in dietetic products for infants: A commentary by the ESPGHAN Committee on Nutrition. J. Paediatr. Gastroenterol. Nutr. 2004, 38, 365–374. [Google Scholar] [CrossRef]
- Van der Aa, L.; Heymans, H.; Van Aalderen, W.; Smitt, J.S.; Knol, J.; Amor, K.B.; Goossens, D.; Sprikkelman, A. Effect of a new synbiotic mixture on atopic dermatitis in infants: A randomized-controlled trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Van der Aa, L.; Van Aalderen, W.; Heymans, H.; Smitt, J.H.S.; Nauta, A.; Knippels, L.; Amor, K.B.; Sprikkelman, A. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.R.; Sladkevicius, E.; Panca, M.; Lack, G.; Guest, J.F. Cost-effectiveness of using an extensively hydrolysed formula compared to an amino acid formula as first-line treatment for cow milk allergy in the UK. Pediatric Allergy Immunol. 2012, 23, 240–449. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W. Effectiveness of using an extensively hydrolyzed casein formula supplemented with Lactobacillus rhamnosus GG compared with an extensively hydrolysed whey formula in managing cow’s milk protein allergic infants. J. Comp. Eff. Res. 2019, 8, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutricia. Neocate Syneo. Available online: https://www.nutricia.co.uk/hcp/pim-products/neocate-syneo.html (accessed on 24 February 2021).
- Nestle Health Science. SMA® Alfamino®. Available online: https://www.nestlehealthscience.co.uk/brands/althera-and-alfamino/alfamino-hcp (accessed on 24 February 2021).
- Mansournia, M.A.; Etminan, M.; Danaei, G.; Kaufman, J.S.; Collins, G. Handling time varying confounding in observational research. Br. Med. J. 2017, 359, j4587. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G.M. Time-varying covariates and coefficients in Cox regression models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef] [Green Version]


| Resource | Cost Per Infant Per Item |
|---|---|
| AAF-Syn (per 400 g tin) [32] | £24.82 |
| AAF (per 400 g tin) [32] | £22.98 |
| Antibiotics (amoxicillin) a | £0.83 |
| Dermatological (soft paraffin) a | £3.76 |
| Anti-reflux (ranitidine) a | £0.83 |
| Dietitian contact b | £92 |
| Other specialist (consultant paediatrician) contact c | £237 |
| Hospital admission d | £577.33 |
| Characteristic | AAF-Syn | AAF |
|---|---|---|
| Sex, n (%) | ||
| Male | 39 (53%) | 40 (54%) |
| Female | 35 (47%) | 34 (46%) |
| IMD quintile, n (%) | ||
| 5th | 27 (36%) | 16 (22%) |
| 4th | 14 (19%) | 18 (24%) |
| 3rd | 12 (16%) | 11 (15%) |
| 2nd | 11 (15%) | 9 (12%) |
| 1st | 10 (14%) | 20 (27%) |
| Mean age at presentation of first symptoms associated with CMPA (months) | 0.56 ± 0.30 | 0.48 ± 0.27 |
| Mean age at CMPA diagnosis/first HAF prescription (months) | 4.71 ± 2.48 | 4.67 ± 2.56 |
| Mean duration on all HAF prescriptions (months) | 10.59 ± 6.82 | 13.69 ± 9.36 |
| Mean duration on the AAF-Syn/AAF (months) | 6.65 ± 5.30 * | 8.44 ± 5.62 * |
| Mean prescription of the AAF-Syn/AAF (g/day) | 148 ± 78 * | 134 ± 89 * |
| Proportion of infants with presenting symptoms before CMPA diagnosis, n (%) | ||
| GI | 31 (42%) | 27 (36%) |
| Skin | 17 (23%) | 18 (24%) |
| Respiratory | 0 (0%) | 2 (2.7%) |
| Proportion of infants with multiple systems affected (GI/respiratory/skin), n (%) | ||
| <2 systems affected | 65 (88%) | 67 (91%) |
| ≥2 systems affected | 9 (12%) | 7 (10%) |
| Proportion of infants with infections before CMPA diagnosis, n (%) | 32 (43%) | 29 (39%) |
| Proportion of infants with other allergy †, n (%) | 9 (12%) | 12 (16%) |
| Proportion of infants with faltering growth before CMPA diagnosis, n (%) | 1 (1.4%) | 2 (2.7%) |
| Proportion of infants with eHF prescription, n (%) | 53 (72%) | 54 (73%) |
| Mean number of allergic medication prescriptions ‡ before CMPA diagnosis | 10.50 ± 7.61 | 10.68 ± 7.51 |
| Mean observation period (years) | 1.19 ± 0.57 | 1.19 ± 0.57 |
| Outcome | AAF-Syn (n = 74) | AAF (n = 74) | p-Value |
|---|---|---|---|
| Symptoms | 2.43 | 3.88 | <0.001 |
| Infections | 1.82 | 2.81 | <0.001 |
| Medication prescriptions | 16.08 | 19.81 | <0.001 |
| Healthcare contacts | 1.17 | 1.43 | 0.15 |
| AAF-Syn (n = 74) | AAF (n = 74) | p-Value | ||
|---|---|---|---|---|
| Symptoms | ||||
| GI symptoms | Proportion | 23% | 46% | 0.006 |
| Rate | 0.43 | 0.72 | 0.013 | |
| Skin symptoms | Proportion | 11% | 26% | 0.033 |
| Rate | 0.17 | 0.32 | 0.066 | |
| Respiratory symptoms | Proportion | 0% | 2.7% | 0.5 |
| Rate | 0 | 0.02 | 0.5 | |
| Infections | ||||
| GI infections | Proportion | 0 | 7% | 0.058 |
| Rate | 0 | 0.10 | 0.004 | |
| Skin infections | Proportion | 26% | 36% | 0.2 |
| Rate | 0.26 | 0.44 | 0.056 | |
| Respiratory infections | Proportion | 59% | 84% | 0.002 |
| Rate | 1.46 | 2.16 | <0.001 | |
| Ear infections | Proportion | 11% | 11% | >0.9 |
| Rate | 0.10 | 0.10 | >0.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorensen, K.; Cawood, A.L.; Cooke, L.H.; Acosta-Mena, D.; Stratton, R.J. The Use of an Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy—Effect on Clinical Outcomes. Nutrients 2021, 13, 2205. https://doi.org/10.3390/nu13072205
Sorensen K, Cawood AL, Cooke LH, Acosta-Mena D, Stratton RJ. The Use of an Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy—Effect on Clinical Outcomes. Nutrients. 2021; 13(7):2205. https://doi.org/10.3390/nu13072205
Chicago/Turabian StyleSorensen, Katy, Abbie L. Cawood, Lisa H. Cooke, Dionisio Acosta-Mena, and Rebecca J. Stratton. 2021. "The Use of an Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy—Effect on Clinical Outcomes" Nutrients 13, no. 7: 2205. https://doi.org/10.3390/nu13072205
APA StyleSorensen, K., Cawood, A. L., Cooke, L. H., Acosta-Mena, D., & Stratton, R. J. (2021). The Use of an Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy—Effect on Clinical Outcomes. Nutrients, 13(7), 2205. https://doi.org/10.3390/nu13072205