Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment
Abstract
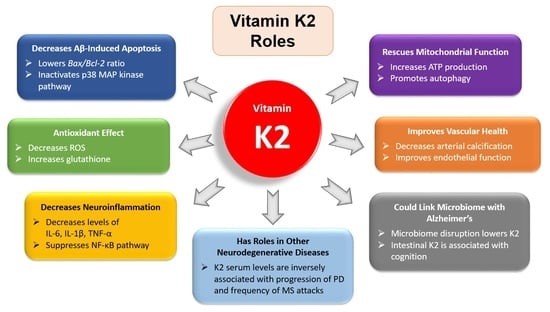
:1. Introduction
2. Comparison of Vitamins K1 and K2
3. Vitamin K2 and Alzheimer’s Disease
3.1. The Antiapoptotic and Antioxidant Effects of Vitamin K2
3.2. Vitamin K2 and Neuroinflammation
3.3. Vitamin K2 and Mitochondrial Dysfunction
3.4. Vitamin K2 and Anesthesia-Induced Cognitive Deficits
3.5. Vitamin K2 and Cardiovascular Health
3.6. Vitamin K2 and the Gut Microbiome
3.7. Vitamin K2 and Comorbidities in Alzheimer’s Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2021 Alzheimer’s Disease Facts and Figures. Available online: www.alz:media/documents/alzheimers-facts-and-figures.pdf (accessed on 27 May 2021).
- Alzheimer’s Disease International; Patterson, C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018. [Google Scholar]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Janssen, J.C.; Beck, J.A.; Campbell, T.A.; Dickinson, A.; Fox, N.C.; Harvey, R.J.; Houlden, H.; Rossor, M.N.; Collinge, J. Early Onset Familial Alzheimer’s Disease: Mutation Frequency in 31 Families. Neurology 2003, 60, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.A., III; Gamez, N.; Escobedo, G., Jr.; Calderon, O.; Moreno-Gonzalez, I. Modifiable Risk Factors for Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.R. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beulens, J.W.J.; Booth, S.L.; van den Heuvel, E.G.H.M.; Stoecklin, E.; Baka, A.; Vermeer, C. The Role of Menaquinones (Vitamin K₂) in Human Health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Uwano, M.; Yamaguchi, N.; Uenishi, K.; Ishida, H.; Sasaki, S.; Okano, T. Vitamin K Content of Foods and Dietary Vitamin K Intake in Japanese Young Women. J. Nutr. Sci. Vitaminol. 2007, 53, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearer, M.J.; Newman, P. Metabolism and Cell Biology of Vitamin K. Thromb. Haemost. 2008, 100, 530–547. [Google Scholar]
- Schurgers, L.J.; Vermeer, C. Determination of Phylloquinone and Menaquinones in Food. Pathophysiol. Haemost. Thromb. 2000, 30, 298–307. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients 2020, 12, 1852. [Google Scholar] [CrossRef]
- Marles, R.J.; Roe, A.L.; Oketch-Rabah, H.A. US Pharmacopeial Convention Safety Evaluation of Menaquinone-7, a Form of Vitamin K. Nutr. Rev. 2017, 75, 553–578. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, Y.; Guo, H.; Sagare, A.; Fernandez, J.A.; Bell, R.D.; Barrett, T.M.; Griffin, J.H.; Freeman, R.S.; Zlokovic, B.V. Protein S Protects Neurons from Excitotoxic Injury by Activating the TAM Receptor Tyro3-Phosphatidylinositol 3-Kinase-Akt Pathway through Its Sex Hormone-Binding Globulin-Like Region. J. Neurosci. 2010, 30, 15521–15534. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, H.; Griffin, J.H.; Fernández, J.A.; Zlokovic, B.V. Protein S Confers Neuronal Protection during Ischemic/Hypoxic Injury in Mice. Circulation 2003, 107, 1791–1796. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wang, Y.; Singh, I.; Bell, R.D.; Deane, R.; Zhong, Z.; Sagare, A.; Winkler, E.A.; Zlokovic, B.V. Protein S Controls Hypoxic/Ischemic Blood-Brain Barrier Disruption through the TAM Receptor Tyro3 and Sphingosine 1-Phosphate Receptor. Blood 2010, 115, 4963–4972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiely, A.; Ferland, G.; Ouliass, B.; O’Toole, P.W.; Purtill, H.; O’Connor, E.M. Vitamin K Status and Inflammation Are Associated with Cognition in Older Irish Adults. Nutr. Neurosci. 2020, 23, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Carrié, I.; Bélanger, E.; Portoukalian, J.; Rochford, J.; Ferland, G. Lifelong Low-Phylloquinone Intake Is Associated with Cognitive Impairments in Old Rats. J. Nutr. 2011, 141, 1495–1501. [Google Scholar] [CrossRef] [Green Version]
- Ferland, G. Vitamin K, an Emerging Nutrient in Brain Function. BioFactors 2012, 38, 151–157. [Google Scholar] [CrossRef]
- Presse, N.; Shatenstein, B.; Kergoat, M.-J.; Ferland, G. Low Vitamin K Intakes in Community-Dwelling Elders at an Early Stage of Alzheimer’s Disease. J. Am. Diet. Assoc. 2008, 108, 2095–2099. [Google Scholar] [CrossRef]
- Presse, N.; Belleville, S.; Gaudreau, P.; Greenwood, C.E.; Kergoat, M.-J.; Morais, J.A.; Payette, H.; Shatenstein, B.; Ferland, G. Vitamin K Status and Cognitive Function in Healthy Older Adults. Neurobiol. Aging 2013, 34, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Soutif-Veillon, A.; Ferland, G.; Rolland, Y.; Presse, N.; Boucher, K.; Féart, C.; Annweiler, C. Increased Dietary Vitamin K Intake Is Associated with Less Severe Subjective Memory Complaint among Older Adults. Maturitas 2016, 93, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Chouet, J.; Ferland, G.; Féart, C.; Rolland, Y.; Presse, N.; Boucher, K.; Barberger-Gateau, P.; Beauchet, O.; Annweiler, C. Dietary Vitamin K Intake Is Associated with Cognition and Behaviour among Geriatric Patients: The CLIP Study. Nutrients 2015, 7, 6739–6750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alisi, L.; Cao, R.; De Angelis, C.; Cafolla, A.; Caramia, F.; Cartocci, G.; Librando, A.; Fiorelli, M. The Relationships between Vitamin K and Cognition: A Review of Current Evidence. Front. Neurol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Van den Heuvel, E.G.H.M.; van Schoor, N.M.; Vermeer, C.; Zwijsen, R.M.L.; den Heijer, M.; Comijs, H.C. Vitamin K Status Is Not Associated with Cognitive Decline in Middle Aged Adults. J. Nutr. Health Aging 2015, 19, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Ferland, G.; Feart, C.; Presse, N.; Lorrain, S.; Bazin, F.; Helmer, C.; Berr, C.; Annweiler, C.; Rouaud, O.; Dartigues, J.-F.; et al. Vitamin K Antagonists and Cognitive Function in Older Adults: The Three-City Cohort Study. J. Gerontol. Ser. A. Biol. Sci. Med. Sci. 2016, 71, 1356–1362. [Google Scholar] [CrossRef] [Green Version]
- Brangier, A.; Celle, S.; Roche, F.; Beauchet, O.; Ferland, G.; Annweiler, C. Use of Vitamin K Antagonists and Brain Morphological Changes in Older Adults: An Exposed/Unexposed Voxel-Based Morphometric Study. Dement. Geriatr. Cogn. Disord. 2018, 45, 18–26. [Google Scholar] [CrossRef]
- Alisi, L.; Cafolla, C.; Gentili, A.; Tartaglione, S.; Curini, R.; Cafolla, A. Vitamin K Concentration and Cognitive Status in Elderly Patients on Anticoagulant Therapy: A Pilot Study. J. Aging Res. 2020, 2020, 9695324. [Google Scholar] [CrossRef] [Green Version]
- Annweiler, C.; Ferland, G.; Barberger-Gateau, P.; Brangier, A.; Rolland, Y.; Beauchet, O. Vitamin K Antagonists and Cognitive Impairment: Results From a Cross-Sectional Pilot Study Among Geriatric Patients. J. Gerontol. Ser. A 2015, 70, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brangier, A.; Ferland, G.; Rolland, Y.; Gautier, J.; Féart, C.; Annweiler, C. Vitamin K Antagonists and Cognitive Decline in Older Adults: A 24-Month Follow-Up. Nutrients 2018, 10, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denisova, N.A.; Booth, S.L. Vitamin K and Sphingolipid Metabolism: Evidence to Date. Nutr. Rev. 2005, 63, 111–121. [Google Scholar] [CrossRef]
- Olsen, A.S.B.; Færgeman, N.J. Sphingolipids: Membrane Microdomains in Brain Development, Function and Neurological Diseases. Open Biol. 2017, 7, 170069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinini, M.; Scandroglio, F.; Prioni, S.; Buccinnà, B.; Loberto, N.; Aureli, M.; Chigorno, V.; Lupino, E.; DeMarco, G.; Lomartire, A.; et al. Deregulated Sphingolipid Metabolism and Membrane Organization in Neurodegenerative Disorders. Mol. Neurobiol. 2010, 41, 314–340. [Google Scholar] [CrossRef]
- Popescu, D.C.; Huang, H.; Singhal, N.K.; Shriver, L.; McDonough, J.; Clements, R.J.; Freeman, E.J. Vitamin K Enhances the Production of Brain Sulfatides during Remyelination. PLoS ONE 2018, 13, e0203057. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla Protein Is an Independent Predictor of Both Intimal and Medial Vascular Calcification in Chronic Kidney Disease. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef] [Green Version]
- Guedes, J.A.C.; Esteves, J.V.; Morais, M.R.; Zorn, T.M.; Furuya, D.T. Osteocalcin Improves Insulin Resistance and Inflammation in Obese Mice: Participation of White Adipose Tissue and Bone. Bone 2018, 115, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Saravanan, S.; d’Adesky, N.D.; Bramlett, H.; Perez-Pinzon, M.A.; Raval, A.P. Osteocalcin, Ovarian Senescence, and Brain Health. Front. Neuroendocrinol. 2020, 59, 100861. [Google Scholar] [CrossRef]
- Shan, C.; Ghosh, A.; Guo, X.; Wang, S.; Hou, Y.; Li, S.; Liu, J. Roles for Osteocalcin in Brain Signalling: Implications in Cognition- and Motor-Related Disorders. Mol. Brain 2019, 12, 23. [Google Scholar] [CrossRef]
- Bellan, M.; Pirisi, M.; Sainaghi, P. The Gas6/TAM System and Multiple Sclerosis. Int. J. Mol. Sci. 2016, 17, 1807. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, Z.; Hu, W.; Wang, D.; Gong, B.; Fan, C.; Jiang, S.; Li, T.; Gao, J.; Yang, Y. Molecular Insights of Gas6/TAM in Cancer Development and Therapy. Cell Death Dis. 2017, 8, e2700. [Google Scholar] [CrossRef]
- Ichikawa, T.; Horie-Inoue, K.; Ikeda, K.; Blumberg, B.; Inoue, S. Vitamin K2 Induces Phosphorylation of Protein Kinase A and Expression of Novel Target Genes in Osteoblastic Cells. J. Mol. Endocrinol. 2007, 39, 239–247. [Google Scholar] [CrossRef]
- Hadipour, E.; Tayarani-Najaran, Z.; Fereidoni, M. Vitamin K2 Protects PC12 Cells against Aβ (1-42) and H2O2-Induced Apoptosis via P38 MAP Kinase Pathway. Nutr. Neurosci. 2020, 23, 343–352. [Google Scholar] [CrossRef]
- Huang, S.-H.; Fang, S.-T.; Chen, Y.-C. Molecular Mechanism of Vitamin K2 Protection against Amyloid-β-Induced Cytotoxicity. Biomolecules 2021, 11, 423. [Google Scholar] [CrossRef]
- Kusano, J.; Tanaka, S.; Matsuda, H.; Hara, Y.; Fujii, Y.; Suzuki, S.; Sekiyama, M.; Ando, E.; Sugiyama, K.; Hirano, T. Vitamin K1 and Vitamin K2 Immunopharmacological Effects on the Peripheral Lymphocytes of Healthy Subjects and Dialysis Patients, as Estimated by the Lymphocyte Immunosuppressant Sensitivity Test. J. Clin. Pharm. Ther. 2018, 43, 895–902. [Google Scholar] [CrossRef]
- Meng, K.; Xu, W.; Miura, T.; Suzuki, S.; Chiyotanda, M.; Tanaka, S.; Sugiyama, K.; Kawashima, H.; Hirano, T. The Effects of Vitamin K1 and Vitamin K2 on the Proliferation, Cytokine Production and Regulatory T-Cell Frequency in Peripheral Blood Mononuclear Cells of Paediatric Atopic Dermatitis Patients. Exp. Dermatol. 2018, 27, 1058–1060. [Google Scholar] [CrossRef]
- Myneni, V.; Mezey, E. Immunomodulatory Effect of Vitamin K2: Implications for Bone Health. Oral Dis. 2018, 24, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Saputra, W.D.; Aoyama, N.; Komai, M.; Shirakawa, H. Menaquinone-4 Suppresses Lipopolysaccharide-Induced Inflammation in MG6 Mouse Microglia-Derived Cells by Inhibiting the NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 2317. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Weitzmann, M.N. Vitamin K2 Stimulates Osteoblastogenesis and Suppresses Osteoclastogenesis by Suppressing NF-ΚB Activation. Int. J. Mol. Med. 2011, 27, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, F.; Mei, C.; Yang, L.; Zheng, J.; Lu, H.; Xia, Y.; Hsu, S.; Liang, H.; Hong, L. Vitamin K2 Promotes PI3K/AKT/HIF-1α-Mediated Glycolysis That Leads to AMPK-Dependent Autophagic Cell Death in Bladder Cancer Cells. Sci. Rep. 2020, 10, 7714. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Tsuchida, A.; Miyazawa, K.; Yokoyama, T.; Kawakita, H.; Tokita, H.; Naito, M.; Itoh, M.; Ohyashiki, K.; Aoki, T. Vitamin K2-Induced Cell Growth Inhibition via Autophagy Formation in Cholangiocellular Carcinoma Cell Lines. Int. J. Mol. Med. 2007, 20, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, S.; Moriya, S.; Kokuba, H.; Hino, H.; Takano, N.; Miyazawa, K. Vitamin K2 Induces Non-Apoptotic Cell Death along with Autophagosome Formation in Breast Cancer Cell Lines. Breast Cancer 2020, 27, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Miyazawa, K.; Naito, M.; Toyotake, J.; Tauchi, T.; Itoh, M.; Yuo, A.; Hayashi, Y.; Georgescu, M.-M.; Kondo, Y.; et al. Vitamin K2 Induces Autophagy and Apoptosis Simultaneously in Leukemia Cells. Autophagy 2008, 4, 629–640. [Google Scholar] [CrossRef]
- Nimptsch, K.; Rohrmann, S.; Linseisen, J. Dietary Intake of Vitamin K and Risk of Prostate Cancer in the Heidelberg Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J. Clin. Nutr. 2008, 87, 985–992. [Google Scholar] [CrossRef]
- Yagami, T. Gas6 Rescues Cortical Neurons from Amyloid β Protein-Induced Apoptosis. Neuropharmacology 2002, 43, 1289–1296. [Google Scholar] [CrossRef]
- Hanzel, C.E.; Pichet-Binette, A.; Pimentel, L.S.B.; Iulita, M.F.; Allard, S.; Ducatenzeiler, A.; Do Carmo, S.; Cuello, A.C. Neuronal Driven Pre-Plaque Inflammation in a Transgenic Rat Model of Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Aman, Y.; Ahmed, I.; Chetelat, G.; Landeau, B.; Ray Chaudhuri, K.; Brooks, D.J.; Edison, P. Influence of Microglial Activation on Neuronal Function in Alzheimer’s and Parkinson’s Disease Dementia. Alzheimers Dement. J. Alzheimer’s Assoc. 2015, 11, 608–621.e7. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Sánchez-Sarasúa, S.; Fernández-Pérez, I.; Espinosa-Fernández, V.; Sánchez-Pérez, A.M.; Ledesma, J.C. Can We Treat Neuroinflammation in Alzheimer’s Disease? Int. J. Mol. Sci. 2020, 21, 8751. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of Microglia and Astrocytes: A Roadway to Neuroinflammation and Alzheimer’s Disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Hanslik, K.L.; Ulland, T.K. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer’s Disease. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kokiko-Cochran, O.N.; Godbout, J.P. The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front. Immunol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Faden, A.I.; Loane, D.J. Chronic Neurodegeneration after Traumatic Brain Injury: Alzheimer Disease, Chronic Traumatic Encephalopathy, or Persistent Neuroinflammation? Neurother. J. Am. Soc. Exp. Neurother. 2015, 12, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.K.; Younkin, S.; et al. TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollingworth, P.; Harold, D.; Sims, R.; Gerrish, A.; Lambert, J.-C.; Carrasquillo, M.M.; Abraham, R.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; et al. Common Variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP Are Associated with Alzheimer’s Disease. Nat. Genet. 2011, 43, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemonnot, A.-L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019, 11, 233. [Google Scholar] [CrossRef] [Green Version]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.-S.; Knapp, D.J.; Crews, F.T. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.A.; Spangenberg, E.E.; Yamate-Morgan, H.; Lee, R.J.; Arora, R.P.S.; Hernandez, M.X.; Tenner, A.J.; West, B.L.; Green, K.N. Elimination of Microglia Improves Functional Outcomes Following Extensive Neuronal Loss in the Hippocampus. J. Neurosci. 2015, 35, 9977–9989. [Google Scholar] [CrossRef]
- Spangenberg, E.E.; Lee, R.J.; Najafi, A.R.; Rice, R.A.; Elmore, M.R.P.; Blurton-Jones, M.; West, B.L.; Green, K.N. Eliminating Microglia in Alzheimer’s Mice Prevents Neuronal Loss without Modulating Amyloid-β Pathology. Brain J. Neurol. 2016, 139 Pt 4, 1265–1281. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.-Y.; Pan, J.-Y.; Chen, Y.; Li, Y.; Wu, J.; Wang, X.-D. Menaquinone-7 Protects Astrocytes by Regulating Mitochondrial Function and Inflammatory Response under Hypoxic Conditions. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10181–10193. [Google Scholar] [CrossRef]
- Agrawal, I.; Jha, S. Mitochondrial Dysfunction and Alzheimer’s Disease: Role of Microglia. Front. Aging Neurosci. 2020, 12, 252. [Google Scholar] [CrossRef]
- De Oliveira, L.G.; Angelo, Y.D.S.; Iglesias, A.H.; Peron, J.P.S. Unraveling the Link between Mitochondrial Dynamics and Neuroinflammation. Front. Immunol. 2021, 12, 624919. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R.; et al. Vitamin K2 Is a Mitochondrial Electron Carrier That Rescues Pink1 Deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Wen, X.; Wei, Z.; Guo, K.; Shi, F.; Huang, T.; Wang, W.; Zheng, J. Vitamin K2 Protects against Aβ42-Induced Neurotoxicity by Activating Autophagy and Improving Mitochondrial Function in Drosophila. Neuroreport 2021, 32, 431–437. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-Modified Gold-Palladium Nanoparticles as a Potential Autophagy Inducer for the Treatment of Alzheimer’s Disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Yamamoto, S.; Ting, T.; Fan, Y.; Sadleir, K.; Wang, Y.; Zhang, W.; Huang, S.; Levine, B.; Vassar, R.; et al. A Becn1 Mutation Mediates Hyperactive Autophagic Sequestration of Amyloid Oligomers and Improved Cognition in Alzheimer’s Disease. PLoS Genet. 2017, 13, e1006962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegh, C.; Pupulin, S.; Wear, D.; Culmone, L.; Huggard, R.; Ma, D.; Pandey, S. Resumption of Autophagy by Ubisol-Q10 in Presenilin-1 Mutated Fibroblasts and Transgenic AD Mice: Implications for Inhibition of Senescence and Neuroprotection. Oxid. Med. Cell. Longev. 2019, 2019, 7404815. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Dong, Y.; Zhang, Y.; Zheng, H.; Shen, Y.; Crosby, G.; Culley, D.J.; Marcantonio, E.R.; Xie, Z. Anesthetic Isoflurane or Desflurane Plus Surgery Differently Affects Cognitive Function in Alzheimer’s Disease Transgenic Mice. Mol. Neurobiol. 2018, 55, 5623–5638. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Tan, H.; Boukhali, M.; Khatri, A.; Yu, Y.; Hua, F.; Liu, L.; Li, M.; Yang, G.; et al. Tau Contributes to Sevoflurane-Induced Neurocognitive Impairment in Neonatal Mice. Anesthesiology 2020, 133, 595–610. [Google Scholar] [CrossRef]
- Bos, D.; Vernooij, M.W.; Elias-Smale, S.E.; Verhaaren, B.F.J.; Vrooman, H.A.; Hofman, A.; Niessen, W.J.; Witteman, J.C.M.; van der Lugt, A.; Ikram, M.A. Atherosclerotic Calcification Relates to Cognitive Function and to Brain Changes on Magnetic Resonance Imaging. Alzheimer’s Dement. 2012, 8, S104–S111. [Google Scholar] [CrossRef] [Green Version]
- Bos, D.; Vernooij, M.W.; de Bruijn, R.F.A.G.; Koudstaal, P.J.; Hofman, A.; Franco, O.H.; van der Lugt, A.; Ikram, M.A. Atherosclerotic Calcification Is Related to a Higher Risk of Dementia and Cognitive Decline. Alzheimers Dement. J. Alzheimer’s Assoc. 2015, 11, 639–647.e1. [Google Scholar] [CrossRef] [Green Version]
- Weimar, C.; Winkler, A.; Dlugaj, M.; Lehmann, N.; Hennig, F.; Bauer, M.; Kröger, K.; Kälsch, H.; Mahabadi, A.-A.; Dragano, N.; et al. Ankle-Brachial Index but Neither Intima Media Thickness Nor Coronary Artery Calcification Is Associated With Mild Cognitive Impairment. J. Alzheimer’s Dis. JAD 2015, 47, 433–442. [Google Scholar] [CrossRef]
- Di Daniele, N.; Celotto, R.; Alunni Fegatelli, D.; Gabriele, M.; Rovella, V.; Scuteri, A. Common Carotid Artery Calcification Impacts on Cognitive Function in Older Patients. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2019, 26, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Vonder, M.; Sidorenkov, G.; Ma, R.; Oudkerk, M.; van der Harst, P.; De Deyn, P.P.; Vliegenthart, R. Coronary Artery Calcium and Cognitive Function in Dutch Adults: Cross-Sectional Results of the Population-Based ImaLife Study. J. Am. Heart Assoc. 2021, 10. [Google Scholar] [CrossRef]
- Cui, C.; Sekikawa, A.; Kuller, L.H.; Lopez, O.L.; Newman, A.B.; Kuipers, A.L.; Mackey, R.H. Aortic Stiffness Is Associated with Increased Risk of Incident Dementia in Older Adults. J. Alzheimer’s Dis. 2018, 66, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Fitzpatrick, A.L.; Lopez, O.; Jackson, S.; Lyketsos, C.; Jagust, W.; Ives, D.; Dekosky, S.T.; Kuller, L.H. Dementia and Alzheimer’s Disease Incidence in Relationship to Cardiovascular Disease in the Cardiovascular Health Study Cohort. J. Am. Geriatr. Soc. 2005, 53, 1101–1107. [Google Scholar] [CrossRef]
- Qiu, C.; Winblad, B.; Viitanen, M.; Fratiglioni, L. Pulse Pressure and Risk of Alzheimer Disease in Persons Aged 75 Years and Older: A Community-Based, Longitudinal Study. Stroke 2003, 34, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Toledo, J.B.; Toledo, E.; Weiner, M.W.; Jack, C.R.; Jagust, W.; Lee, V.M.Y.; Shaw, L.M.; Trojanowski, J.Q. Alzheimer’s Disease Neuroimaging Initiative. Cardiovascular Risk Factors, Cortisol, and Amyloid-β Deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2012, 8, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Grinberg, L.T.; Thal, D.R. Vascular Pathology in the Aged Human Brain. Acta Neuropathol. 2010, 119, 277–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaria, R.N.; Akinyemi, R.; Ihara, M. Does Vascular Pathology Contribute to Alzheimer Changes? J. Neurol. Sci. 2012, 322, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Kapasi, A.; Schneider, J.A. Vascular Contributions to Cognitive Impairment, Clinical Alzheimer’s Disease, and Dementia in Older Persons. Biochim. Biophys. Acta 2016, 1862, 878–886. [Google Scholar] [CrossRef]
- Kelleher, R.J.; Soiza, R.L. Evidence of Endothelial Dysfunction in the Development of Alzheimer’s Disease: Is Alzheimer’s a Vascular Disorder? Am. J. Cardiovasc. Dis. 2013, 3, 197–226. [Google Scholar] [PubMed]
- De la Torre, J.C. Vascular Risk Factor Detection and Control May Prevent Alzheimer’s Disease. Ageing Res. Rev. 2010, 9, 218–225. [Google Scholar] [CrossRef]
- De Bruijn, R.F.; Ikram, M.A. Cardiovascular Risk Factors and Future Risk of Alzheimer’s Disease. BMC Med. 2014, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-F.; Smith, A.V.; Aspelund, T.; Betensky, R.A.; Smoller, J.W.; Gudnason, V.; Launer, L.J.; Blacker, D. Genetic Overlap between Vascular Pathologies and Alzheimer’s Dementia and Potential Causal Mechanisms. Alzheimers Dement. J. Alzheimer’s Assoc. 2019, 15, 65–75. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.A. The Overlap between Vascular Disease and Alzheimer’s Disease-Lessons from Pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef] [Green Version]
- Jellinger, K.A. Alzheimer Disease and Cerebrovascular Pathology: An Update. J. Neural Transm. 2002, 109, 813–836. [Google Scholar] [CrossRef]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of Cerebrovascular Disease in Autopsy Confirmed Neurodegenerative Disease Cases in the National Alzheimer’s Coordinating Centre. Brain J. Neurol. 2013, 136 Pt 9, 2697–2706. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.J.; van der Meer, I.M.; Hofman, A.; Witteman, J.C.M. Dietary Intake of Menaquinone Is Associated with a Reduced Risk of Coronary Heart Disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef]
- Haugsgjerd, T.R.; Egeland, G.M.; Nygård, O.K.; Vinknes, K.J.; Sulo, G.; Lysne, V.; Igland, J.; Tell, G.S. Association of Dietary Vitamin K and Risk of Coronary Heart Disease in Middle-Age Adults: The Hordaland Health Study Cohort. BMJ Open 2020, 10, e035953. [Google Scholar] [CrossRef]
- Gast, G.C.M.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.J.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.M.; van der Schouw, Y.T. A High Menaquinone Intake Reduces the Incidence of Coronary Heart Disease. Nutr. Metab. Cardiovasc. Dis. NMCD 2009, 19, 504–510. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Bots, M.L.; Atsma, F.; Bartelink, M.-L.E.L.; Prokop, M.; Geleijnse, J.M.; Witteman, J.C.M.; Grobbee, D.E.; van der Schouw, Y.T. High Dietary Menaquinone Intake Is Associated with Reduced Coronary Calcification. Atherosclerosis 2009, 203, 489–493. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Braam, L.A.J.L.M.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.G.; Vermeer, C. Menaquinone-7 Supplementation Improves Arterial Stiffness in Healthy Postmenopausal Women. A Double-Blind Randomised Clinical Trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Zwakenberg, S.R.; de Jong, P.A.; Bartstra, J.W.; van Asperen, R.; Westerink, J.; de Valk, H.; Slart, R.H.J.A.; Luurtsema, G.; Wolterink, J.M.; de Borst, G.J.; et al. The Effect of Menaquinone-7 Supplementation on Vascular Calcification in Patients with Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Zwakenberg, S.R.; den Braver, N.R.; Engelen, A.I.P.; Feskens, E.J.M.; Vermeer, C.; Boer, J.M.A.; Verschuren, W.M.M.; van der Schouw, Y.T.; Beulens, J.W.J. Vitamin K Intake and All-Cause and Cause Specific Mortality. Clin. Nutr. 2017, 36, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Intake of Vitamin K Is Inversely Associated with Mortality Risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Spronk, H.M.H.; Soute, B.A.M.; Schurgers, L.J.; Thijssen, H.H.W.; De Mey, J.G.R.; Vermeer, C. Tissue-Specific Utilization of Menaquinone-4 Results in the Prevention of Arterial Calcification in Warfarin-Treated Rats. J. Vasc. Res. 2003, 40, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Kus, K.; Manterys, A.; Proniewski, B.; Sternak, M.; Przyborowski, K.; Moorlag, M.; Sitek, B.; Marczyk, B.; Jasztal, A.; et al. Vitamin K2-MK-7 Improves Nitric Oxide-Dependent Endothelial Function in ApoE/LDLR-/- Mice. Vascul. Pharmacol. 2019, 122–123, 106581. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Kanekiyo, T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal Microbiota Transplantation Alleviated Alzheimer’s Disease-like Pathogenesis in APP/PS1 Transgenic Mice. Transl. Psychiatry 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Holsinger, R.M.D.; Elangovan, S. Neuroprotective Effects of Fecal Microbiota Transplantation in a Mouse Model of Alzheimer’s Disease: Development of New Models and Analysis Methods/Validation of Pre-clinical Methods. Alzheimer’s Dement. 2020, 16. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a Healthy Microbiota Reduces Amyloid and Tau Pathology in an Alzheimer’s Disease Animal Model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal Metabolite of a Gnotobiotic Mouse Transplanted with Gut Microbiota from a Patient with Alzheimer’s Disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Hazan, S. Rapid Improvement in Alzheimer’s Disease Symptoms Following Fecal Microbiota Transplantation: A Case Report. J. Int. Med. Res. 2020, 48, 030006052092593. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-Induced Perturbations in Gut Microbial Diversity Influences Neuro-Inflammation and Amyloidosis in a Murine Model of Alzheimer’s Disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta Amyloid Pathology in APPPS1 Transgenic Mice in the Absence of Gut Microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Corsini, S.; Kellingray, L.; Hegarty, C.; Gall, G.L.; Narbad, A.; Müller, M.; Tejera, N.; O’Toole, P.W.; Minihane, A.-M.; et al. APOE Genotype Influences the Gut Microbiome Structure and Function in Humans and Mice: Relevance for Alzheimer’s Disease Pathophysiology. FASEB J. 2019, 33, 8221–8231. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic Effect of Fructooligosaccharides from Morinda Officinalis on Alzheimer’s Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [Green Version]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Brandscheid, C.; Schuck, F.; Reinhardt, S.; Schäfer, K.-H.; Pietrzik, C.U.; Grimm, M.; Hartmann, T.; Schwiertz, A.; Endres, K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2017, 56, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Bäuerl, C.; Collado, M.C.; Diaz Cuevas, A.; Viña, J.; Pérez Martínez, G. Shifts in Gut Microbiota Composition in an APP/PSS1 Transgenic Mouse Model of Alzheimer’s Disease during Lifespan. Lett. Appl. Microbiol. 2018, 66, 464–471. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.-F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimer’s Dis. JAD 2017, 56, 385–390. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.-F.; Huang, L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2017, 60, 1241–1257. [Google Scholar] [CrossRef]
- Cox, L.M.; Schafer, M.J.; Sohn, J.; Vincentini, J.; Weiner, H.L.; Ginsberg, S.D.; Blaser, M.J. Calorie Restriction Slows Age-Related Microbiota Changes in an Alzheimer’s Disease Model in Female Mice. Sci. Rep. 2019, 9, 17904. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-L.; Li, W.-W.; Wang, J.; Xu, Y.-L.; Sun, H.-L.; Tian, D.-Y.; Wang, Y.-J.; Yao, X.-Q. Gut Microbiota Alteration and Its Time Course in a Tauopathy Mouse Model. J. Alzheimer’s Dis. JAD 2019, 70, 399–412. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, L.J.; Zhang, L.J. Neuroinflammation, Gut Microbiome, and Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8243–8250. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—a Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Van Olst, L.; Roks, S.J.M.; Kamermans, A.; Verhaar, B.J.H.; van der Geest, A.M.; Muller, M.; van der Flier, W.M.; de Vries, H.E. Contribution of Gut Microbiota to Immunological Changes in Alzheimer’s Disease. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, T.; Takagishi, Y.; Uchida, K.; Yamada, H. Colonic Absorption of Menaquinone-4 and Menaquinone-9 in Rats. J. Nutr. 1992, 122, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Conly, J.M.; Stein, K. Quantitative and Qualitative Measurements of K Vitamins in Human Intestinal Contents. Am. J. Gastroenterol. 1992, 87, 311–316. [Google Scholar]
- Conly, J.M. Assay of Menaquinones in Bacterial Cultures, Stool Samples, and Intestinal Contents. Methods Enzymol. 1997, 282, 457–466. [Google Scholar] [CrossRef]
- Suttie, J.W. The Importance of Menaquinones in Human Nutrition. Annu. Rev. Nutr. 1995, 15, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Bach, A.; Kohlmeier, M. Chemistry, Nutritional Sources, Tissue Distribution and Metabolism of Vitamin K with Special Reference to Bone Health. J. Nutr. 1996, 126 (Suppl. S4), 1181S–1186S. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kakuya, F.; Ito, S. Vitamin K1 and K2 Status and Faecal Flora in Breast Fed and Formula Fed 1-Month-Old Infants. Eur. J. Pediatr. 1993, 152, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Guss, J.D.; Taylor, E.; Rouse, Z.; Roubert, S.; Higgins, C.H.; Thomas, C.J.; Baker, S.P.; Vashishth, D.; Donnelly, E.; Shea, M.K.; et al. The Microbial Metagenome and Bone Tissue Composition in Mice with Microbiome-Induced Reductions in Bone Strength. Bone 2019, 127, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Sheh, A.; Ellis, J.L.; Smith, D.E.; Booth, S.L.; Fu, X.; Muthupalani, S.; Ge, Z.; Puglisi, D.A.; Wang, T.C.; et al. Helicobacter Pylori Antibiotic Eradication Coupled with a Chemically Defined Diet in INS-GAS Mice Triggers Dysbiosis and Vitamin K Deficiency Resulting in Gastric Hemorrhage. Gut Microbes 2020, 11, 820–841. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Pompili, M.; Di Stasio, E.; Zocco, M.A.; Gasbarrini, A.; Flore, R. Subclinical Atherosclerosis Is Linked to Small Intestinal Bacterial Overgrowth via Vitamin K2-Dependent Mechanisms. World J. Gastroenterol. 2017, 23, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- McCann, A.; Jeffery, I.B.; Ouliass, B.; Ferland, G.; Fu, X.; Booth, S.L.; Tran, T.T.T.; O’Toole, P.W.; O’Connor, E.M. Exploratory Analysis of Covariation of Microbiota-Derived Vitamin K and Cognition in Older Adults. Am. J. Clin. Nutr. 2019, 110, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Ruiz, J.I.; García-Cobián, T.A.; Pascoe-González, S.; Sánchez-Enríquez, S.; Llamas-Covarrubias, I.M.; García-Iglesias, T.; López-Quintero, A.; Llamas-Covarrubias, M.A.; Trujillo-Quiroz, J.; Rivera-Leon, E.A. Effect of Supplementation with Vitamins D3 and K2 on Undercarboxylated Osteocalcin and Insulin Serum Levels in Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Clinical Trial. Diabetol. Metab. Syndr. 2020, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Rahimi Sakak, F.; Moslehi, N.; Niroomand, M.; Mirmiran, P. Glycemic Control Improvement in Individuals with Type 2 Diabetes with Vitamin K2 Supplementation: A Randomized Controlled Trial. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Yu, J.; Choi, H.; An, J.H.; Kim, S.W.; Park, K.S.; Jang, H.C.; Kim, S.Y.; Shin, C.S. Vitamin K2 Supplementation Improves Insulin Sensitivity via Osteocalcin Metabolism: A Placebo-Controlled Trial. Diabetes Care 2011, 34, e147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beulens, J.W.; Grobbee, D.E.; Sluijs, I.; Spijkerman, A.M.; Van Der Schouw, Y.T. Dietary Phylloquinone and Menaquinones Intakes and Risk of Type 2 Diabetes. Diabetes Care 2010, 33, 1699–1705. [Google Scholar] [CrossRef] [Green Version]
- Dam, V.; Dalmeijer, G.W.; Vermeer, C.; Drummen, N.E.; Knapen, M.H.; van der Schouw, Y.T.; Beulens, J.W. Association Between Vitamin K and the Metabolic Syndrome: A 10-Year Follow-Up Study in Adults. J. Clin. Endocrinol. Metab. 2015, 100, 2472–2479. [Google Scholar] [CrossRef] [Green Version]
- Dash, N.R.; Al Bataineh, M.T. Metagenomic Analysis of the Gut Microbiome Reveals Enrichment of Menaquinones (Vitamin K2) Pathway in Diabetes Mellitus. Diabetes Metab. J. 2021, 45, 77–85. [Google Scholar] [CrossRef]
- Hussein, A.G.; Mohamed, R.H.; Shalaby, S.M.; Abd El Motteleb, D.M. Vitamin K2 Alleviates Type 2 Diabetes in Rats by Induction of Osteocalcin Gene Expression. Nutrition 2018, 47, 33–38. [Google Scholar] [CrossRef]
- Desentis-Desentis, M.F.; Rivas-Carrillo, J.D.; Sánchez-Enríquez, S. Protective Role of Osteocalcin in Diabetes Pathogenesis. J. Bone Miner. Metab. 2020, 38, 765–771. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine Regulation of Energy Metabolism by the Skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Shen, L.; Ji, H.-F. Alzheimer’s Disease and Risk of Hip Fracture: A Meta-Analysis Study. Sci. World J. 2012, 2012, e872173. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-K.; Hung, C.-M.; Lin, S.-H.; Tai, Y.-C.; Lu, K.; Liliang, P.-C.; Lin, C.-W.; Lee, Y.-C.; Fang, P.-H.; Chang, L.-C.; et al. Increased Risk of Hip Fractures in Patients with Dementia: A Nationwide Population-Based Study. BMC Neurol. 2014, 14, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; Lo, R.Y. Alzheimer’s Disease and Osteoporosis. Tzu-Chi Med. J. 2017, 29, 138–142. [Google Scholar] [CrossRef]
- Amouzougan, A.; Lafaie, L.; Marotte, H.; Dẻnariẻ, D.; Collet, P.; Pallot-Prades, B.; Thomas, T. High Prevalence of Dementia in Women with Osteoporosis. Jt. Bone Spine 2017, 84, 611–614. [Google Scholar] [CrossRef]
- Zhou, R.; Deng, J.; Zhang, M.; Zhou, H.-D.; Wang, Y.-J. Association between Bone Mineral Density and the Risk of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2011, 24, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Chung, C.-J.; Lin, C.-L.; Sung, F.-C.; Wu, T.-N.; Kao, C.-H. Increased Risk of Dementia in Patients with Osteoporosis: A Population-Based Retrospective Cohort Analysis. Age Dordr. Neth. 2014, 36, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koitaya, N.; Sekiguchi, M.; Tousen, Y.; Nishide, Y.; Morita, A.; Yamauchi, J.; Gando, Y.; Miyachi, M.; Aoki, M.; Komatsu, M.; et al. Low-Dose Vitamin K2 (MK-4) Supplementation for 12 Months Improves Bone Metabolism and Prevents Forearm Bone Loss in Postmenopausal Japanese Women. J. Bone Miner. Metab. 2014, 32, 142–150. [Google Scholar] [CrossRef]
- Purwosunu, Y.; Muharram; Rachman, I.A.; Reksoprodjo, S.; Sekizawa, A. Vitamin K2 Treatment for Postmenopausal Osteoporosis in Indonesia. J. Obstet. Gynaecol. Res. 2006, 32, 230–234. [Google Scholar] [CrossRef]
- Ikeda, Y.; Iki, M.; Morita, A.; Kajita, E.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. Intake of Fermented Soybeans, Natto, Is Associated with Reduced Bone Loss in Postmenopausal Women: Japanese Population-Based Osteoporosis (JPOS) Study. J. Nutr. 2006, 136, 1323–1328. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Drummen, N.E.; Smit, E.; Vermeer, C.; Theuwissen, E. Three-Year Low-Dose Menaquinone-7 Supplementation Helps Decrease Bone Loss in Healthy Postmenopausal Women. Osteoporos. Int. J. 2013, 24, 2499–2507. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Schurgers, L.J.; Vermeer, C. Vitamin K2 Supplementation Improves Hip Bone Geometry and Bone Strength Indices in Postmenopausal Women. Osteoporos. Int. J. 2007, 18, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Rønn, S.H.; Harsløf, T.; Pedersen, S.B.; Langdahl, B.L. Vitamin K2 (Menaquinone-7) Prevents Age-Related Deterioration of Trabecular Bone Microarchitecture at the Tibia in Postmenopausal Women. Eur. J. Endocrinol. 2016, 175, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Herbert, J.; Lucassen, P.J. Depression as a Risk Factor for Alzheimer’s Disease: Genes, Steroids, Cytokines and Neurogenesis-What Do We Need to Know? Front. Neuroendocrinol. 2016, 41, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Spalletta, G.; Caltagirone, C.; Girardi, P.; Gianni, W.; Casini, A.R.; Palmer, K. The Role of Persistent and Incident Major Depression on Rate of Cognitive Deterioration in Newly Diagnosed Alzheimer’s Disease Patients. Psychiatry Res. 2012, 198, 263–268. [Google Scholar] [CrossRef]
- Modrego, P.J. Depression in Alzheimer’s Disease. Pathophysiology, Diagnosis, and Treatment. J. Alzheimer’s Dis. JAD 2010, 21, 1077–1087. [Google Scholar] [CrossRef]
- Gancheva, S.M.; Zhelyazkova-Savova, M.D. Vitamin K2 Improves Anxiety and Depression but Not Cognition in Rats with Metabolic Syndrome: A Role of Blood Glucose? Folia Med. 2016, 58, 264–272. [Google Scholar] [CrossRef]
- NIA-Funded Active Alzheimer’s and Related Dementias Clinical Trials and Studies. Available online: www.nia.nih.gov/research/ongoing-AD-trials (accessed on 27 May 2021).
- PubMed Search Results of Clinical Trials Using Vitamin K2. Available online: pubmed.ncbi.nlm.nih.gov/?term=vitamin+K2&filter=pubt.clinicaltrial (accessed on 27 May 2021).
- Lasemi, R.; Kundi, M.; Moghadam, N.B.; Moshammer, H.; Hainfellner, J.A. Vitamin K2 in Multiple Sclerosis Patients. Wien. Klin. Wochenschr. 2018, 130, 307–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.-X.; Yu, X.-D.; Cheng, Q.-Z.; Tang, L.; Shen, M.-Q. The Association of Serum Vitamin K2 Levels with Parkinson’s Disease: From Basic Case-Control Study to Big Data Mining Analysis. Aging 2020, 12, 16410–16419. [Google Scholar] [CrossRef] [PubMed]




| Experimental Group | Percent of Apoptotic Cells (%) |
|---|---|
| Control for Aβ(1–42) | 3.2 |
| 20 mcmol K2 + Aβ(1–42) | 4.3 |
| Aβ(1–42) Only | 13.1 |
| Control for H2O2 | 4.7 |
| 20 mcmol K2 + H2O2 | 6.4 |
| H2O2 Only | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, A.; German, M. Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment. Nutrients 2021, 13, 2206. https://doi.org/10.3390/nu13072206
Popescu A, German M. Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment. Nutrients. 2021; 13(7):2206. https://doi.org/10.3390/nu13072206
Chicago/Turabian StylePopescu, Alexander, and Monica German. 2021. "Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment" Nutrients 13, no. 7: 2206. https://doi.org/10.3390/nu13072206
APA StylePopescu, A., & German, M. (2021). Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment. Nutrients, 13(7), 2206. https://doi.org/10.3390/nu13072206