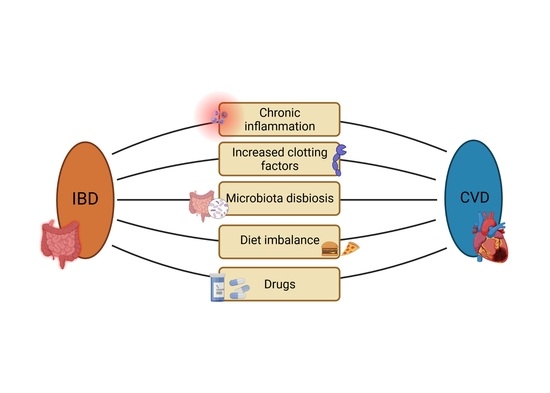
What Links an Increased Cardiovascular Risk and Inflammatory Bowel Disease? A Narrative Review
Abstract
:1. Introduction
2. Cardiovascular Risk in Patients with IBD
2.1. IBD and Ischaemic Heart Disease
2.2. IBD and Venous Thromboembolic Events
2.2.1. Venous Thromboembolism Incidents in IBD
2.2.2. Arterial Thromboembolism and Cerebrovascular Events in IBD
3. Risk Factors for CVD in IBD Patients
3.1. Inflammation
3.2. Drugs Used in IBD and CV Risk
3.3. Gut Microbiota in IBD and CVD
4. Dietary Support, Supplementation and Imaging Techniques
4.1. Mediterranean Diet
4.1.1. Mediterranean Diet in CVD
4.1.2. Mediterranean Diet in IBD
4.2. Western-Style Diet
4.2.1. Western-Style Diet in CVD
4.2.2. Western-Style Diet in IBD
4.3. Calcium and Vitamin D in CVD and IBD
4.4. Imaging Techniques and IBD
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pac-Kożuchowska, E.; Krawiec, P.; Mroczkowska-Juchkiewicz, A.; Pawłowska-Kamieniak, A.; Kominek, K. Inflammatory and lipid-associated markers of cardiovascular diseases in children with first exacerbation of inflammatory bowel disease. Med. Sci. Monit. 2016, 22, 1534–1539. [Google Scholar] [CrossRef]
- Panhwar, M.S.; Mansoor, E.; Al-Kindi, S.G.; Sinh, P.; Katz, J.; Oliveira, G.H.; Cooper, G.S.; Ginwalla, M. Risk of myocardial infarction in inflammatory bowel disease: A population-based national study. Inflamm. Bowel Dis. 2019, 25, 1080–1087. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Rev. Esp. Cardiol. 2016, 69, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Jensen, G.V.; Torp-Pedersen, C.; Nielsen, O.H.; Gislason, G.H.; Hansen, P.R. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death—A Danish nationwide cohort study. PLoS ONE 2013, 8, e56944. [Google Scholar] [CrossRef]
- Singh, S.; Singh, H.; Loftus, E.V.; Pardi, D.S. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 382–393.e1. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Beaugerie, L.; Carrat, F.; Andersen, N.N.; Jess, T.; Schwarzinger, M.; BERENICE Study Group. Increased risk of acute arterial events in young patients and severely active IBD: A nationwide French cohort study. Gut 2018, 67, 1261–1268. [Google Scholar] [CrossRef]
- Rungoe, C.; Basit, S.; Ranthe, M.F.; Wohlfahrt, J.; Langholz, E.; Jess, T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut 2013, 62, 689–694. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation and atherosclerosis. Circulation 2017, 136, 1875–1877. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [Green Version]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L.; et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Verdecchia, P.; Reboldi, G.; Angeli, F.; Trimarco, B.; Mancia, G.; Pogue, J.; Gao, P.; Sleight, P.; Teo, K.; Yusuf, S. Systolic and diastolic blood pressure changes in relation with myocardial infarction and stroke in patients with coronary artery disease. Hypertension 2015, 65, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Aniwan, S.; Pardi, D.S.; Tremaine, W.J.; Loftus, E.V. Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1607–1615.e1. [Google Scholar] [CrossRef] [Green Version]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Cappello, M.; Licata, A.; Calvaruso, V.; Bravatà, I.; Aiello, A.; Torres, D.; Della Corte, V.; Tuttolomondo, A.; Perticone, M.; Licata, G.; et al. Increased expression of markers of early atherosclerosis in patients with inflammatory bowel disease. Eur. J. Intern. Med. 2017, 37, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Grainge, M.J.; West, J.; Card, T.R. Venous thromboembolism during active disease and remission in inflammatory bowel disease: A cohort study. Lancet 2010, 375, 657–663. [Google Scholar] [CrossRef]
- Solomon, D.H.; Reed, G.W.; Kremer, J.M.; Curtis, J.R.; Farkouh, M.E.; Harrold, L.R.; Hochberg, M.C.; Tsao, P.; Greenberg, J.D. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015, 67, 1449–1455. [Google Scholar] [CrossRef]
- Agca, R.; Smulders, Y.; Nurmohamed, M. Cardiovascular disease risk in immune-mediated inflammatory diseases: Recommendations for clinical practice. Heart 2021. [Google Scholar] [CrossRef]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in IBD. Expert Opin. Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, frailty and cardiovascular disease. Adv. Exp. Med. Biol. 2020, 1216, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Binesh, A.; Devaraj, S.N.; Halagowder, D. Molecular Interaction of NFκB and NICD in monocyte-macrophage differentiation is a target for intervention in atherosclerosis. J. Cell. Physiol. 2019, 234, 7040–7050. [Google Scholar] [CrossRef]
- Das, S.; Zhang, E.; Senapati, P.; Amaram, V.; Reddy, M.A.; Stapleton, K.; Leung, A.; Lanting, L.; Wang, M.; Chen, Z.; et al. A Novel angiotensin ii-induced long noncoding RNA giver regulates oxidative stress, inflammation, and proliferation in vascular smooth muscle cells. Circ. Res. 2018, 123, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.P.; Chung, A.; Yan, B.P.; Gibson, P.R. Venous and arterial disease in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2013, 28, 1095–1113. [Google Scholar] [CrossRef]
- Chung, W.-S.; Lin, C.-L.; Hsu, W.-H.; Kao, C.-H. Inflammatory bowel disease increases the risks of deep vein thrombosis and pulmonary embolism in the hospitalized patients: A nationwide cohort study. Thromb. Res. 2015, 135, 492–496. [Google Scholar] [CrossRef]
- Aggarwal, A.; Atreja, A.; Kapadia, S.; Lopez, R.; Achkar, J.-P. Conventional risk factors and cardiovascular outcomes of patients with inflammatory bowel disease with confirmed coronary artery disease. Inflamm. Bowel Dis. 2015, 21, E2. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Bernstein, C.N.; Bitton, A.; Chan, A.K.; Griffiths, A.M.; Leontiadis, G.I.; Geerts, W.; Bressler, B.; Butzner, J.D.; Carrier, M.; et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 2014, 146, 835–848.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, N.E.; Harrison, N.; Junga, Z.; Singla, M. Heart under attack: Cardiac manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2018, 24, 2322–2326. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Zuliani, G.; Rigatelli, G.; Favero, G.D.; Roncon, L. Atrial fibrillation in patients with inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Intern. Med. 2020, 76, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Pattanshetty, D.J.; Anna, K.; Gajulapalli, R.D.; Sappati-Biyyani, R.R. Inflammatory bowel “cardiac” disease: Point prevalence of atrial fibrillation in inflammatory bowel disease population. Saudi J. Gastroenterol. 2015, 21, 325–329. [Google Scholar] [CrossRef]
- Vizzardi, E.; Sciatti, E.; Bonadei, I.; Bordonali, T.; Ricci, C.; Lanzarotto, F.; Lanzini, A.; Metra, M. Subclinical cardiac involvement in Crohn’s disease and ulcerative colitis: An echocardiographic case-control study. Panminerva Med. 2016, 58, 115–120. [Google Scholar]
- Tsai, M.-S.; Lin, C.-L.; Chen, H.-P.; Lee, P.-H.; Sung, F.-C.; Kao, C.-H. Long-term risk of acute coronary syndrome in patients with inflammatory bowel disease: A 13-year nationwide cohort study in an Asian population. Inflamm. Bowel Dis. 2014, 20, 502–507. [Google Scholar] [CrossRef]
- Sridhar, A.R.M.; Parasa, S.; Navaneethan, U.; Crowell, M.D.; Olden, K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J. Crohns Colitis 2011, 5, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Sinh, P.; Tabibian, J.H.; Biyani, P.S.; Mehta, K.; Mansoor, E.; Loftus, E.V.; Dave, M. Inflammatory bowel disease does not impact mortality but increases length of hospitalization in patients with acute myocardial infarction. Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef]
- Pemmasani, G.; Elgendy, I.; Mamas, M.A.; Leighton, J.A.; Aronow, W.S.; Tremaine, W.J. Epidemiology and clinical outcomes of patients with inflammatory bowel disease presenting with acute coronary syndrome. Inflamm. Bowel Dis. 2021, 27, 1017–1025. [Google Scholar] [CrossRef]
- Card, T.R.; Zittan, E.; Nguyen, G.C.; Grainge, M.J. Disease activity in inflammatory bowel disease is associated with arterial vascular disease. Inflamm. Bowel Dis. 2021, 27, 629–638. [Google Scholar] [CrossRef]
- Kobo, O.; Mohamed, M.O.; Farmer, A.D.; Alraies, C.M.; Patel, T.; Sharma, K.; Nolan, J.; Bagur, R.; Roguin, A.; Mamas, M.A. Outcomes of Percutaneous Coronary Intervention in Patients with Crohn’s disease and ulcerative colitis (from a nationwide cohort). Am. J. Cardiol. 2020, 130, 30–36. [Google Scholar] [CrossRef]
- Dogan, Y.; Soylu, A.; Eren, G.A.; Poturoglu, S.; Dolapcioglu, C.; Sonmez, K.; Duman, H.; Sevindir, I. Evaluation of QT and P wave dispersion and mean platelet volume among inflammatory bowel disease patients. Int. J. Med. Sci. 2011, 8, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Schöttker, B.; Herder, C.; Rothenbacher, D.; Roden, M.; Kolb, H.; Müller, H.; Brenner, H. Proinflammatory cytokines, adiponectin, and increased risk of primary cardiovascular events in diabetic patients with or without renal dysfunction. Diabetes Care 2013, 36, 1703–1711. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Jiang, L.; Xu, L.; Tian, J.; Xu, Y.; Zhao, Y.; Feng, X.; Wu, Y.; Zhang, Y.; Wang, D.; et al. Predictive value of in-hospital white blood cell count in chinese patients with triple-vessel coronary disease. Eur. J. Prev. Cardiol. 2019, 26, 872–882. [Google Scholar] [CrossRef]
- Leppkes, M.; Neurath, M.F. Cytokines in inflammatory bowel diseases—Update 2020. Pharmacol. Res. 2020, 158, 104835. [Google Scholar] [CrossRef]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Danese, S.; Sans, M.; Scaldaferri, F.; Sgambato, A.; Rutella, S.; Cittadini, A.; Piqué, J.M.; Panes, J.; Katz, J.A.; Gasbarrini, A.; et al. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: A novel anti-inflammatory mechanism of infliximab in Crohn’s disease. J. Immunol. 2006, 176, 2617–2624. [Google Scholar] [CrossRef]
- Zanoli, L.; Ozturk, K.; Cappello, M.; Inserra, G.; Geraci, G.; Tuttolomondo, A.; Torres, D.; Pinto, A.; Duminuco, A.; Riguccio, G.; et al. Inflammation and aortic pulse wave velocity: A multicenter longitudinal study in patients with inflammatory bowel disease. JAHA 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Schwager, S.; Detmar, M. Inflammation and lymphatic function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Testa, U.; Pannitteri, G.; Condorelli, G.L. Vascular endothelial growth factors in cardiovascular medicine. J. Cardiovasc. Med. 2008, 9, 1190–1221. [Google Scholar] [CrossRef]
- Wang, A.; Liu, J.; Li, C.; Gao, J.; Li, X.; Chen, S.; Wu, S.; Ding, H.; Fan, H.; Hou, S. Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of cardiovascular disease. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Pujades-Rodriguez, M.; Morgan, A.W.; Cubbon, R.M.; Wu, J. Dose-dependent Oral Glucocorticoid Cardiovascular Risks in People with Immune-Mediated Inflammatory Diseases: A population-based cohort study. PLoS Med. 2020, 17, e1003432. [Google Scholar] [CrossRef]
- Singh, J.A.; Wells, G.A.; Christensen, R.; Tanjong Ghogomu, E.; Maxwell, L.; Macdonald, J.K.; Filippini, G.; Skoetz, N.; Francis, D.; Lopes, L.C.; et al. Adverse effects of biologics: A network meta-analysis and cochrane overview. Cochrane Database Syst. Rev. 2011, CD008794. [Google Scholar] [CrossRef]
- Atzeni, F.; Nucera, V.; Galloway, J.; Zoltán, S.; Nurmohamed, M. Cardiovascular risk in ankylosing spondylitis and the effect of anti-TNF drugs: A narrative review. Expert Opin. Biol. Ther. 2020, 20, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Bigeh, A.; Sanchez, A.; Maestas, C.; Gulati, M. Inflammatory bowel disease and the risk for cardiovascular disease: Does all inflammation lead to heart disease? Trends Cardiovasc. Med. 2020, 30, 463–469. [Google Scholar] [CrossRef]
- Palm, H.-G.; Bauer, A.; Weinerth, J.; Wiedmann, K.H. Sinusvenenthrombose als komplikation einer floriden colitis ulcerosa. Med. Klin. 2006, 101, 659–661. [Google Scholar] [CrossRef]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, V.R.; Petrosino, J.F.; Bryan, R.M., Jr.; Durgan, D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgart, M.; Dogan, B.; Rishniw, M.; Weitzman, G.; Bosworth, B.; Yantiss, R.; Orsi, R.H.; Wiedmann, M.; McDonough, P.; Kim, S.G.; et al. Culture independent analysis of Ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of clostridiales in Crohn’s disease involving the ileum. ISME J. 2007, 1, 403–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, R.B. Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc. Natl. Acad. Sci. USA 2008, 105, 16413–16414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Liao, S.; He, Y.; Wang, S.; Xia, G.; Liu, F.; Zhu, J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of gut microbiota with reduced trimethylamine-n-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. JAHA 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Kashtanova, D.; Tkacheva, O.; Popenko, A.; Egshatyan, L.; Tyakht, A.; Alexeev, D.; Kotovskaya, Y.; Plokhova, E.; Boytsov, S. Gut microbiota and vascular biomarkers in patients without clinical cardiovascular diseases. Artery Res. 2017, 18, 41. [Google Scholar] [CrossRef]
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B.; et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J. Vasc. Surg. 2018, 68, 1552–1562.e7. [Google Scholar] [CrossRef]
- Huć, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2018, 130, 172–179. [Google Scholar] [CrossRef]
- Denning, T.L.; Wang, Y.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17–producing t cell responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef]
- Barrington, W.T.; Lusis, A.J. Association between the gut microbiome and atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 699–700. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of bifidobacterium and the lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Jafarabadi, M.A.; Hedayati, M.; Ghavami, A.; Alipour, S.; Alamdari, N.M.; Barati, M.; Ostadrahimi, A. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: A randomized double-blind, placebo-controlled trial. Horm. Metab. Res. 2017, 49, 886–891. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin ii-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin–Angiotensin system. J. Hypertens. 2017, 35, 1899–1908. [Google Scholar] [CrossRef]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.S.; Chan, Q.; Ebbels, T.; De Iorio, M.; Brown, I.J.; Veselkov, K.A.; et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar] [CrossRef]
- Knock, G.; Psaroudakis, D.; Abbot, S.; Aaronson, P.I. Propionate-induced relaxation in rat mesenteric arteries: A role for endothelium-derived hyperpolarising factor. J. Physiol. 2002, 538, 879–890. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.N.; Capettini, L.S.A.; Lemos, V.S.; Santos, R.A.S.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [Green Version]
- Santino, A.; Scarano, A.; De Santis, S.; De Benedictis, M.; Giovinazzo, G.; Chieppa, M. Gut microbiota modulation and anti-inflammatory properties of dietary polyphenols in IBD: New and consolidated perspectives. Curr. Pharmacol. Des. 2017, 23, 2344–2351. [Google Scholar] [CrossRef]
- Haskey, N.; Gibson, D.L. An examination of diet for the maintenance of remission in inflammatory bowel disease. Nutrients 2017, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary therapy with the crohn’s disease exclusion diet is a successful strategy for induction of remission in children and adults failing biological therapy. J. Crohns Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Barkas, F.; Nomikos, T.; Liberopoulos, E.; Panagiotakos, D. Diet and cardiovascular disease risk among individuals with familial hypercholesterolemia: Systematic review and meta-analysis. Nutrients 2020, 12, 2436. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.-M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef] [Green Version]
- Baigent, C.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; Backer, G.G.D.; Delgado, V.; Ference, B.A.; Graham, I.M.; Halliday, A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar]
- Liyanage, T.; Ninomiya, T.; Wang, A.; Neal, B.; Jun, M.; Wong, M.G.; Jardine, M.; Hillis, G.S.; Perkovic, V. Effects of the Mediterranean diet on cardiovascular outcomes—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0159252. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Mistretta, A.; Frigiola, A.; Gruttadauria, S.; Biondi, A.; Basile, F.; Vitaglione, P.; D’Orazio, N.; Galvano, F. Mediterranean diet and cardiovascular risk factors: A systematic review. Crit. Rev. Food Sci. Nutr. 2014, 54, 593–610. [Google Scholar] [CrossRef]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.-A.; Covas, M.-I.; Lamuela-Raventos, M.R.; Salas-Salvadó, J.; Fiol, M.; et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [Green Version]
- Gardener, H.; Wright, C.B.; Cabral, D.; Scarmeas, N.; Gu, Y.; Cheung, K.; Elkind, M.S.V.; Sacco, R.L.; Rundek, T. Mediterranean diet and carotid atherosclerosis in the Northern Manhattan study. Atherosclerosis 2014, 234, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. Ser. A 2018, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Shannon, O.M.; Mendes, I.; Köchl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.-M.; Mathers, J.C.; Siervo, M. Mediterranean diet increases endothelial function in adults: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef]
- Rychter, A.M.; Ratajczak, A.E.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Non-systematic review of diet and nutritional risk factors of cardiovascular disease in obesity. Nutrients 2020, 12, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrdoljak, J.; Vilović, M.; Živković, P.M.; Tadin Hadjina, I.; Rušić, D.; Bukić, J.; Borovac, J.A.; Božić, J. Mediterranean diet adherence and dietary attitudes in patients with inflammatory bowel disease. Nutrients 2020, 12, 3429. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Amerikanou, C.; Forbes, A.; Kaliora, A.C. Adherence to Mediterranean diet in Crohn’s disease. Eur. J. Nutr. 2020, 59, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Cenni, S.; Serra, M.R.; Dolce, P.; Martinelli, M.; Staiano, A.; Miele, E. Effectiveness of Mediterranean diet’s adherence in children with inflammatory bowel diseases. Nutrients 2020, 12, 3206. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory activity of extra virgin olive oil polyphenols: Which role in the prevention and treatment of immune-mediated inflammatory diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean Diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Giugliano, F.; Martinelli, M.; Cenni, S.; Greco, L.; Staiano, A.; Miele, E. Impact of environmental and familial factors in a cohort of pediatric patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 569–574. [Google Scholar] [CrossRef]
- Fung, T.; Rimm, E.; Spiegelman, D.; Rifai, N.; Tofler, G.; Willett, W.; Hu, F. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular risk. Am. J. Clin. Nutr. 2001, 73, 61–67. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and cardiovascular health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [Green Version]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, Metabolites, and “Western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary patterns and risk of inflammatory bowel disease in Europe: Results from the EPIC study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic Review and Meta-Analysis: Association of a pre-illness western dietary pattern with the risk of developing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 362–371. [Google Scholar] [CrossRef]
- Tubbs, A.L.; Liu, B.; Rogers, T.D.; Sartor, R.B.; Miao, E.A. Dietary salt exacerbates experimental colitis. J. Immunol. 2017, 199, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A diet low in red and processed meat does not reduce rate of Crohn’s disease flares. Gastroenterology 2019, 157, 128–136.e5. [Google Scholar] [CrossRef] [Green Version]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm. J. 2019, 23. [Google Scholar] [CrossRef]
- Castro, F.; de Souza, H.S.P. Dietary composition and effects in inflammatory bowel disease. Nutrients 2019, 11, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Triviño, F.J.; Arias-Santiago, S.; Gilaberte-Calzada, Y. Vitamin D and the skin: A review for dermatologists. Actas Dermosifiliogr. 2019, 110, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dardzińska, J.; Banach, D.; Małgorzewicz, S. Plant-based diet and risk for osteoporosis. Forum Zaburzeń Metab. 2016, 7, 99–105. [Google Scholar]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The role of vitamin D in inflammatory bowel disease: Mechanism to management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A.; Pilz, S. Vitamin D and cardiovascular disease: An update. Anticancer Res. 2019, 39, 4627–4635. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–188S. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Rai, V.; Agrawal, D.K. Role of vitamin D in cardiovascular diseases. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1039–1059. [Google Scholar] [CrossRef]
- Nitsa, A.; Toutouza, M.; Machairas, N.; Mariolis, A.; Philippou, A.; Koutsilieris, M. Vitamin D in cardiovascular disease. In Vivo 2018, 32, 977–981. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-Hydroxyvitamin D, ethnicity, and blood pressure in the third national health and nutrition examination survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the third national health and nutrition examination survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Annweiler, C.; Duval, G.; Karras, S.; Tirabassi, G.; Salvio, G.; Balercia, G.; Kimball, S.; Kotsa, K.; Mascitelli, L.; et al. Vitamin D and Cardiovascular Disease: From Atherosclerosis to myocardial infarction and stroke. Int. J. Cardiol. 2017, 230, 577–584. [Google Scholar] [CrossRef]
- Wong, M.S.K.; Delansorne, R.; Man, R.Y.K.; Vanhoutte, P.M. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H289–H296. [Google Scholar] [CrossRef]
- Yin, K.; You, Y.; Swier, V.; Tang, L.; Radwan, M.M.; Pandya, A.N.; Agrawal, D.K. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2432–2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of vitamin D in atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angellotti, E.; D’Alessio, D.; Dawson-Hughes, B.; Chu, Y.; Nelson, J.; Hu, P.; Cohen, R.M.; Pittas, A.G. Effect of vitamin D supplementation on cardiovascular risk in type 2 diabetes. Clin. Nutr. 2019, 38, 2449–2453. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kaźmierczak, I.; Szymczak, A.; Tomczak, M.; Łykowska-Szuber, L.; Linke, K.; Eder, P. Calcium and phosphate metabolism in patients with inflammatory bowel diseases. Pol. Arch. Med. Wewn. 2015, 125, 588–590. [Google Scholar] [CrossRef] [Green Version]
- Bolland, M.J.; Avenell, A.; Baron, J.A.; Grey, A.; MacLennan, G.S.; Gamble, G.D.; Reid, I.R. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 2010, 341, c3691. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Kaaks, R.; Linseisen, J.; Rohrmann, S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the heidelberg cohort of the European prospective investigation into cancer and nutrition study (EPIC-Heidelberg). Heart 2012, 98, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Reid, I.R.; Mason, B.; Horne, A.; Ames, R.; Clearwater, J.; Bava, U.; Orr-Walker, B.; Wu, F.; Evans, M.C.; Gamble, G.D. Effects of calcium supplementation on serum lipid concentrations in normal older women: A randomized controlled trial. Am. J. Med. 2002, 112, 343–347. [Google Scholar] [CrossRef]
- Zaid, M.; Fujiyoshi, A.; Kadota, A.; Abbott, R.D.; Miura, K. Coronary artery calcium and carotid artery intima media thickness and plaque: Clinical use in need of clarification. J. Atheroscler. Thromb. 2017, 24, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kullo, I.J.; Pardi, D.S.; Loftus, E.V. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 26–35. [Google Scholar] [CrossRef]
- Nambi, V.; Chambless, L.; Folsom, A.R.; He, M.; Hu, Y.; Mosley, T.; Volcik, K.; Boerwinkle, E.; Ballantyne, C.M. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (Atherosclerosis Risk In Communities) study. J. Am. Coll. Cardiol. 2010, 55, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Camba, A.; Carrillo-Palau, M.; Ramos, L.; Hernández Alvarez-Buylla, N.; Alonso-Abreu, I.; Hernández-Pérez, A.; Vela, M.; Arranz, L.; Hernández-Guerra, M.; González-Gay, M.Á.; et al. Carotid plaque assessment reclassifies patients with inflammatory bowel disease into very-high cardiovascular risk. J. Clin. Med. 2021, 10, 1671. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łykowska-Szuber, L.; Rychter, A.M.; Dudek, M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Zawada, A.; Eder, P.; Lesiak, M.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Links an Increased Cardiovascular Risk and Inflammatory Bowel Disease? A Narrative Review. Nutrients 2021, 13, 2661. https://doi.org/10.3390/nu13082661
Łykowska-Szuber L, Rychter AM, Dudek M, Ratajczak AE, Szymczak-Tomczak A, Zawada A, Eder P, Lesiak M, Dobrowolska A, Krela-Kaźmierczak I. What Links an Increased Cardiovascular Risk and Inflammatory Bowel Disease? A Narrative Review. Nutrients. 2021; 13(8):2661. https://doi.org/10.3390/nu13082661
Chicago/Turabian StyleŁykowska-Szuber, Liliana, Anna Maria Rychter, Magdalena Dudek, Alicja Ewa Ratajczak, Aleksandra Szymczak-Tomczak, Agnieszka Zawada, Piotr Eder, Maciej Lesiak, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "What Links an Increased Cardiovascular Risk and Inflammatory Bowel Disease? A Narrative Review" Nutrients 13, no. 8: 2661. https://doi.org/10.3390/nu13082661
APA StyleŁykowska-Szuber, L., Rychter, A. M., Dudek, M., Ratajczak, A. E., Szymczak-Tomczak, A., Zawada, A., Eder, P., Lesiak, M., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). What Links an Increased Cardiovascular Risk and Inflammatory Bowel Disease? A Narrative Review. Nutrients, 13(8), 2661. https://doi.org/10.3390/nu13082661