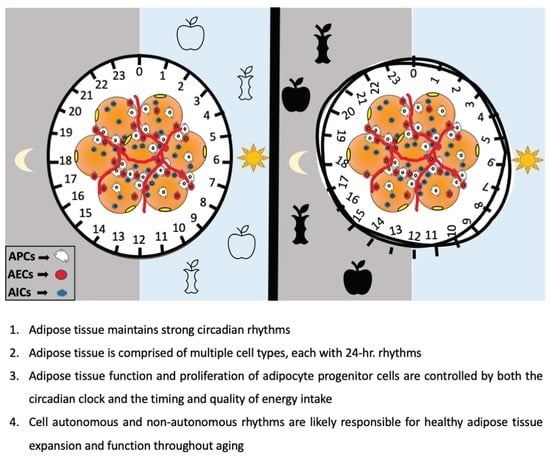
Nutrients and the Circadian Clock: A Partnership Controlling Adipose Tissue Function and Health
Abstract
:1. The Adipose Tissue Circadian Clock
2. The Circadian Clock in the Adipose Stromal Cell Vascular Fraction
2.1. The Circadian Clock in Adipose Progenitor Cells (APCs)
2.2. Endothelial and Immune Cells of the SVF: An Additional Role for the Clock in Adipose Tissue?
3. Nutrients as Zeitgebers in Adipose Tissue
3.1. Effect of High Fat Diet (HFD) in the AT Clock
3.2. Effect of Sugar on the AT Clock
3.3. Effect of Polyphenols in the AT Clock
3.4. Effect of Natural Alkaloids and Passionflower Extract on the AT Clock
3.5. Effect of Endogenous Biomolecules in the AT Clock
3.6. Effect of Xenobiotic Metabolism and Other Endogenous Compounds on the AT Clock
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, V.R.; Ceglia, N.; Zeller, M.; Eckel-Mahan, K.; Sassone-Corsi, P.; Baldi, P. The Pervasiveness and Plasticity of Circadian Oscillations: The Coupled Circadian-Oscillators Framework. Bioinformatics 2015, 31, 3181–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel-Mahan, K.L.; Patel, V.R.; De Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, M.S.; Cox, J.; Mann, M. In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism. PLoS Genet. 2014, 10, e1004047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, M.S.; Humphrey, S.J.; Mann, M. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab. 2017, 25, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.; Patel, V.R.; Eckel-Mahan, K.L.; Peleg, S.; Forne, I.; Ladurner, A.G.; Baldi, P.; Imhof, A.; Sassone-Corsi, P. Circadian Acetylome Reveals Regulation of Mitochondrial Metabolic Pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 3339–3344. [Google Scholar] [CrossRef] [Green Version]
- Green, C.B.; Takahashi, J.S.; Bass, J. The Meter of Metabolism. Cell 2008, 134, 728–742. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.I.; Inoue, I.; Takahashi, S.; Komoda, T.; Katayama, S. Cryptochrome and Period Proteins Are Regulated by the CLOCK/BMAL1 Gene: Crosstalk between the PPARs/RXRalpha-Regulated and CLOCK/BMAL1-Regulated Systems. PPAR Res. 2008, 2008, 348610. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of Nutrient Metabolism and the Circadian Clock System: Importance for Metabolic Health. Mol. Metab. 2016, 5, 133–152. [Google Scholar] [CrossRef]
- Shostak, A.; Meyer-Kovac, J.; Oster, H. Circadian Regulation of Lipid Mobilization in White Adipose Tissues. Diabetes 2013, 62, 2195–2203. [Google Scholar] [CrossRef] [Green Version]
- Zvonic, S.; Ptitsyn, A.A.; Conrad, S.A.; Scott, L.K.; Floyd, Z.E.; Kilroy, G.; Wu, X.; Goh, B.C.; Mynatt, R.L.; Gimble, J.M. Characterization of Peripheral Circadian Clocks in Adipose Tissues. Diabetes 2006, 55, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A Circadian Gene Expression Atlas in Mammals: Implications for Biology and Medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Santos, C.; Gómez-Abellán, P.; Madrid, J.A.; Hernández-Morante, J.J.; Lujan, J.A.; Ordovas, J.M.; Garaulet, M. Circadian Rhythm of Clock Genes in Human Adipose Explants. Obesity 2009, 17, 1481–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas-Latre, A.; Santos, R.B.; Fekry, B.; Tamim, Y.M.; Shivshankar, S.; Mohamed, A.M.T.; Baumgartner, C.; Kwok, C.; Gebhardt, C.; Rivera, A.; et al. Cellular and Physiological Circadian Mechanisms Drive Diurnal Cell Proliferation and Expansion of White Adipose Tissue. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Maury, E.; Navez, B.; Brichard, S.M. Circadian Clock Dysfunction in Human Omental Fat Links Obesity to Metabolic Inflammation. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.; Ribas Latre, A.; Kolonin, M.G. Adipose Stromal Cell Expansion and Exhaustion: Mechanisms and Consequences. Cells 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Knutson, K.L. Consequences of Circadian Disruption on Cardiometabolic Health. Sleep Med. Clin. 2015, 10, 455–468. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose Tissue Remodeling and Obesity. J. Clin. Invest. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [Green Version]
- Petrus, P.; Mejhert, N.; Corrales, P.; Lecoutre, S.; Li, Q.; Maldonado, E.; Kulyté, A.; Lopez, Y.; Campbell, M.; Acosta, J.R.; et al. Transforming Growth Factor-Β3 Regulates Adipocyte Number in Subcutaneous White Adipose Tissue. Cell Rep. 2018, 25, 551–560.e5. [Google Scholar] [CrossRef] [Green Version]
- Kettner, N.M.; Mayo, S.A.; Hua, J.; Lee, C.; Moore, D.D.; Fu, L. Circadian Dysfunction Induces Leptin Resistance In. Cell Metab. 2015, 22, 448–459. [Google Scholar] [CrossRef] [Green Version]
- Shea, S.A.; Hilton, M.F.; Orlova, C.; Timothy Ayers, R.; Mantzoros, C.S. Independent Circadian and Sleep/Wake Regulation of Adipokines and Glucose in Humans. J. Clin. Endocrinol. Metab. 2005, 90, 2537–2544. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 Controls Lipid Metabolism by Direct Regulation of PPARγ. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco-Solis, R.; Aguilar-Arnal, L.; Murakami, M.; Peruquetti, R.; Ramadori, G.; Coppari, R.; Sassone-Corsi, P. The Circadian Clock in the Ventromedial Hypothalamus Controls Cyclic Energy Expenditure. Cell Metab. 2016, 23, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooijman, S.; Van den Berg, R.; Ramkisoensing, A.; Boon, M.R.; Kuipers, E.N.; Loef, M.; Zonneveld, T.C.M.; Lucassen, E.A.; Sips, H.C.M.; Chatzispyrou, I.A.; et al. Prolonged Daily Light Exposure Increases Body Fat Mass through Attenuation of Brown Adipose Tissue Activity. Proc. Natl. Acad. Sci. USA 2015, 112, 6748–6753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onder, Y.; Green, C.B. Rhythms of Metabolism in Adipose Tissue and Mitochondria. Neurobiol. Sleep Circadian Rhythm. 2018, 4, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE Real-Time Reporting of Circadian Dynamics Reveals Persistent Circadian Oscillations in Mouse Peripheral Tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, M.; Kolbe, I.; Seemann, J.; Tsang, A.H.; Cherradi, L.; Klein, J.; Oster, H. Circadian Clock Rhythms in Different Adipose Tissue Model Systems. Chronobiol. Int. 2018, 35, 1543–1552. [Google Scholar] [CrossRef]
- Wu, X.; Zvonic, S.; Floyd, Z.E.; Kilroy, G.; Goh, B.C.; Hernandez, T.L.; Eckel, R.H.; Mynatt, R.L.; Gimble, J.M. Induction of Circadian Gene Expression in Human Subcutaneous Adipose-Derived Stem Cells. Obesity 2007, 15, 2560–2570. [Google Scholar] [CrossRef]
- Aggarwal, A.; Costa, M.J.; Rivero-Gutiérrez, B.; Ji, L.; Morgan, S.L.; Feldman, B.J. The Circadian Clock Regulates Adipogenesis by a Per3 Crosstalk Pathway to Klf15. Cell Rep. 2017, 21, 2367–2375. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Xu, L.; Cai, T.; Yuan, G.; Sun, N.; Lu, C.; Qian, R. Clock Represses Preadipocytes Adipogenesis via GILZ. J. Cell Physiol. 2018, 233, 6028–6040. [Google Scholar] [CrossRef]
- Shi, X.; Shi, W.; Li, Q.; Song, B.; Wan, M.; Bai, S.; Cao, X. A Glucocorticoid-Induced Leucine-Zipper Protein, GILZ, Inhibits Adipogenesis of Mesenchymal Cells. EMBO Rep. 2003, 4, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Gimble, J.M.; Bray, M.S.; Young, A. Circadian Biology and Sleep: Missing Links in Obesity and Metabolism? Obes. Rev. 2009, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Yamaguchi, S.; Mitsui, S.; Emi, A.; Shimoda, F.; Okamura, H. Control Mechanism of the Circadian Clock for Timing of Cell Division in Vivo. Science 2003, 302, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekry, B.; Ribas-Latre, A.; Baumgartner, C.; Deans, J.R.; Kwok, C.; Patel, P.; Fu, L.; Berdeaux, R.; Sun, K.; Kolonin, M.G.; et al. Incompatibility of the Circadian Protein BMAL1 and HNF4α in Hepatocellular Carcinoma. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, J.; Montellier, E.; Sassone-Corsi, P. Molecular Cogs: Interplay between Circadian Clock and Cell Cycle. Trends Cell Biol. 2018, 28, 368–379. [Google Scholar] [CrossRef]
- Christou, S.; Wehrens, S.M.T.; Isherwood, C.; Möller-Levet, C.S.; Wu, H.; Revell, V.L.; Bucca, G.; Skene, D.J.; Laing, E.E.; Archer, S.N.; et al. Circadian Regulation in Human White Adipose Tissue Revealed by Transcriptome and Metabolic Network Analysis. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Bahrami-Nejad, Z.; Zhao, M.L.; Tholen, S.; Hunerdosse, D.; Tkach, K.E.; Van Schie, S.; Chung, M.; Teruel, M.N. A Transcriptional Circuit Filters Oscillating Circadian Hormonal Inputs to Regulate Fat Cell Differentiation. Cell Metab. 2018, 27, 854–868.e8. [Google Scholar] [CrossRef]
- Wang, N.; Yang, G.; Jia, Z.; Zhang, H.; Aoyagi, T.; Soodvilai, S.; Symons, J.D.; Schnermann, J.B.; Gonzalez, F.J.; Litwin, S.E.; et al. Vascular PPARgamma Controls Circadian Variation in Blood Pressure and Heart Rate through Bmal1. Cell Metab. 2008, 8, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Hudson, N.; Celkova, L.; Hopkins, A.; Greene, C.; Storti, F.; Ozaki, E.; Fahey, E.; Theodoropoulou, S.; Kenna, P.F.; Humphries, M.M.; et al. Dysregulated Claudin-5 Cycling in the Inner Retina Causes Retinal Pigment Epithelial Cell Atrophy. JCI Insight 2019, 4, e130273. [Google Scholar] [CrossRef]
- Zhang, S.L.; Lahens, N.F.; Yue, Z.; Arnold, D.M.; Pakstis, P.P.; Schwarz, J.E.; Sehgal, A. A Circadian Clock Regulates Efflux by the Blood-Brain Barrier in Mice and Human Cells. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Takeda, N.; Maemura, K.; Horie, S.; Oishi, K.; Imai, Y.; Harada, T.; Saito, T.; Shiga, T.; Amiya, E.; Manabe, I.; et al. Thrombomodulin Is a Clock-Controlled Gene in Vascular Endothelial Cells. J. Biol. Chem. 2007, 282, 32561–32567. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A Circadian Clock in Macrophages Controls Inflammatory Immune Responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef] [Green Version]
- Druzd, D.; Matveeva, O.; Ince, L.; Harrison, U.; He, W.; Schmal, C.; Herzel, H.; Tsang, A.H.; Kawakami, N.; Leliavski, A.; et al. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 2017, 46, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Clark, G.T.; Yu, Y.; Urban, C.A.; Fu, G.; Wang, C.; Zhang, F.; Linhardt, R.J.; Hurley, J.M. Circadian Control of Heparan Sulfate Levels Times Phagocytosis of Amyloid Beta Aggregates. PLoS Genet. 2022, 18, e1009994. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic Nerves Govern Circadian Leukocyte Recruitment to Tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Holtkamp, S.; Hergenhan, S.M.; Kraus, K.; De Juan, A.; Weber, J.; Bradfield, P.; Grenier, J.M.P.; Pelletier, J.; Druzd, D.; et al. Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures That Guide the Homing of Leukocyte Subsets to Tissues. Immunity 2018, 49, 1175–1190.e7. [Google Scholar] [CrossRef] [Green Version]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; De Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-Wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585.e11. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Neuendorff, N.; Chapkin, R.S.; Earnest, D.J. Role of Inflammatory Signaling in the Differential Effects of Saturated and Poly-Unsaturated Fatty Acids on Peripheral Circadian Clocks. EBioMedicine 2016, 7, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Grygiel-Górniak, B. Peroxisome Proliferator-Activated Receptors and Their Ligands: Nutritional and Clinical Implications—A Review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR Nuclear Receptors as Drug Targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [Green Version]
- La Fleur, S.E.; Luijendijk, M.C.M.; Van der Zwaal, E.M.; Brans, M.A.D.; Adan, R.A.H. The Snacking Rat as Model of Human Obesity: Effects of a Free-Choice High-Fat High-Sugar Diet on Meal Patterns. Int. J. Obes. 2013, 38, 643–649. [Google Scholar] [CrossRef]
- Morris, M.; Araujo, I.C.; Pohlman, R.L.; Marques, M.C.; Rodwan, N.S.; Farah, V.M.A. Timing of Fructose Intake: An Important Regulator of Adiposity. Clin. Exp. Pharmacol. Physiol. 2012, 39, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Bray, M.S.; Young, M.E. Regulation of Fatty Acid Metabolism by Cell Autonomous Circadian Clocks: Time to Fatten up on Information? J. Biol. Chem. 2011, 286, 11883. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.; Portillo, M.P.; Madrid, J.A.; Arias, N.; Macarulla, M.T.; Garaulet, M. Effects of Resveratrol on Changes Induced by High-Fat Feeding on Clock Genes in Rats. Br. J. Nutr. 2013, 110, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Pifferi, F.; Dal-Pan, A.; Languille, S.; Aujard, F. Effects of Resveratrol on Daily Rhythms of Locomotor Activity and Body Temperature in Young and Aged Grey Mouse Lemurs. Oxidative Med. Cell. Longev. 2013, 2013, 187301. [Google Scholar] [CrossRef]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.v.; Lee, J.E.; Yen, E.S.; Lamming, D.W.; et al. Evidence for a Common Mechanism of SIRT1 Regulation by Allosteric Activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Chronic Consumption of Dietary Proanthocyanidins Modulates Peripheral Clocks in Healthy and Obese Rats. J. Nutr. Biochem. 2015, 26, 112–119. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG Ameliorates Diet-Induced Metabolic Syndrome Associating with the Circadian Clock. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oike, H.; Kobori, M.; Suzuki, T.; Ishida, N. Caffeine Lengthens Circadian Rhythms in Mice. Biochem. Biophys. Res. Commun. 2011, 410, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Oishi, K.; Kawano, Y.; Yamazaki, Y. The Harmala Alkaloid Harmine Is a Modulator of Circadian Bmal1 Transcription. Biosci. Rep. 2012, 32, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onishi, Y.; Kawano, Y.; Yamazaki, Y. Lycorine, a Candidate for the Control of Period Length in Mammalian Cells. Cell. Physiol. Biochem. 2012, 29, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimizu, N.; Shimoda, H. Passionflower Extract Induces High-Amplitude Rhythms without Phase Shifts in the Expression of Several Circadian Clock Genes in Vitro and in Vivo. Int. J. Biomed. Sci. 2017, 13, 84–92. [Google Scholar] [PubMed]
- Sakamoto, A.; Terui, Y.; Uemura, T.; Igarashi, K.; Kashiwagi, K. Translational Regulation of Clock Genes BMAL1 and REV-ERBα by Polyamines. Int. J. Mol. Sci. 2021, 22, 1307. [Google Scholar] [CrossRef]
- Zwighaft, Z.; Aviram, R.; Shalev, M.; Rousso-Noori, L.; Kraut-Cohen, J.; Golik, M.; Brandis, A.; Reinke, H.; Aharoni, A.; Kahana, C.; et al. Circadian Clock Control by Polyamine Levels through a Mechanism That Declines with Age. Cell Metab. 2015, 22, 874–885. [Google Scholar] [CrossRef] [Green Version]
- Shirai, H.; Oishi, K.; Ishida, N. Bidirectional CLOCK/BMAL1-Dependent Circadian Gene Regulation by Retinoic Acid in Vitro. Biochem. Biophys. Res. Commun. 2006, 351, 387–391. [Google Scholar] [CrossRef]
- Stehlin-Gaon, C.; Willmann, D.; Zeyer, D.; Sanglier, S.; Van Dorsselaer, A.; Renaud, J.P.; Moras, D.; Schüle, R. All-Trans Retinoic Acid Is a Ligand for the Orphan Nuclear Receptor RORβ. Nat. Struct. Mol. Biol. 2003, 10, 820–825. [Google Scholar] [CrossRef]
- Branecky, K.L.; Niswender, K.D.; Pendergast, J.S. Disruption of Daily Rhythms by High-Fat Diet Is Reversible. PLoS ONE 2015, 10, e0137970. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, H.; Ando, H.; Hayashi, Y.; Obi, Y.; Fujimura, A. High-Fat Feeding Exerts Minimal Effects on Rhythmic MRNA Expression of Clock Genes in Mouse Peripheral Tissues. Chronobiol. Int. 2006, 23, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.S.; Tsai, J.-Y.; Villegas-Montoya, C.; Boland, B.B.; Blasier, Z.; Egbejimi, O.; Kueht, M.; Young, M.E. Time-of-Day-Dependent Dietary Fat Consumption Influences Multiple Cardiometabolic Syndrome Parameters in Mice. Int. J. Obes. 2010, 34, 1589–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschos, G.K.; Ibrahim, S.; Song, W.L.; Kunieda, T.; Grant, G.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in Mice with Adipocyte-Specific Deletion of Clock Component Arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef] [Green Version]
- Fonken, L.K.; Aubrecht, T.G.; Meléndez-Fernández, O.H.; Weil, Z.M.; Nelson, R.J. Dim Light at Night Disrupts Molecular Circadian Rhythms and Increases Body Weight. J. Biol. Rhythm. 2013, 28, 262–271. [Google Scholar] [CrossRef]
- Klyde, B.J.; Hirsch, J. Increased Cellular Proliferation in Adipose Tissue of Adult Rats Fed a High-Fat Diet. J. Lipid Res. 1979, 20, 705–715. [Google Scholar] [CrossRef]
- Shin, S.; Pang, Y.; Park, J.; Liu, L.; Lukas, B.E.; Kim, S.H.; Kim, K.W.; Xu, P.; Berry, D.C.; Jiang, Y. Dynamic Control of Adipose Tissue Development and Adult Tissue Homeostasis by Platelet-Derived Growth Factor Receptor Alpha. eLife 2020, 9, e56189. [Google Scholar] [CrossRef]
- Kalsbeek, A.; La Fleur, S.; Fliers, E. Circadian Control of Glucose Metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Oike, H.; Nagai, K.; Fukushima, T.; Ishida, N.; Kobori, M. Feeding Cues and Injected Nutrients Induce Acute Expression of Multiple Clock Genes in the Mouse Liver. PLoS ONE 2011, 6, e23709. [Google Scholar] [CrossRef] [Green Version]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; Nusinow, D.A.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome Mediates Circadian Regulation of CAMP Signaling and Hepatic Gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Attia, R.R.; Connnaughton, S.; Boone, L.R.; Wang, F.; Elam, M.B.; Ness, G.C.; Cook, G.A.; Park, E.A. Regulation of Pyruvate Dehydrogenase Kinase 4 (PDK4) by Thyroid Hormone Role of the Peroxisome Proliferator-Activated Receptor γ Coactivator (PGC-1α). J. Biol. Chem. 2010, 285, 2375–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Aragonès, G.; Suárez, M.; Ardid-Ruiz, A.; Vinaixa, M.; Rodríguez, M.A.; Correig, X.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Boost Hepatic NAD+ Metabolism and SIRT1 Expression and Activity in a Dose-Dependent Manner in Healthy Rats. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Bladé, C.; Arola, L. Dietary Proanthocyanidins Modulate BMAL1 Acetylation, Nampt Expression and NAD Levels in Rat Liver. Sci. Rep. 2015, 5, 10954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Modulate Melatonin Levels in Plasma and the Expression Pattern of Clock Genes in the Hypothalamus of Rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef]
- Jager, J.; Wang, F.; Fang, B.; Lim, H.W.; Peed, L.C.; Steger, D.J.; Won, K.J.; Kharitonenkov, A.; Adams, A.C.; Lazar, M.A. The Nuclear Receptor Rev-Erbα Regulates Adipose Tissue-Specific FGF21 Signaling. J. Biol. Chem. 2016, 291, 10867–10875. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Rodríguez, A.; Ortega, F.; Becerril, S.; Girones, J.; Sabater-Masdeu, M.; Latorre, J.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Heme Biosynthetic Pathway Is Functionally Linked to Adipogenesis via Mitochondrial Respiratory Activity. Obesity 2017, 25, 1723–1733. [Google Scholar] [CrossRef]
- Freeman, S.L.; Kwon, H.; Portolano, N.; Parkin, G.; Girija, U.V.; Basran, J.; Fielding, A.J.; Fairall, L.; Svistunenko, D.A.; Moody, P.C.E.; et al. Heme Binding to Human CLOCK Affects Interactions with the E-Box. Proc. Natl. Acad. Sci. USA 2019, 116, 19911–19916. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, Z.; Scott Gabrielsen, J.; Simcox, J.A.; Lee, S.H.; Jones, D.; Cooksey, B.; Stoddard, G.; Cefalu, W.T.; McClain, D.A. Adipocyte Iron Regulates Leptin and Food Intake. J. Clin. Investig. 2015, 125, 3681–3691. [Google Scholar] [CrossRef] [Green Version]
- Gau, D.; Lemberger, T.; Von Gall, C.; Kretz, O.; Le Minh, N.; Gass, P.; Schmid, W.; Schibler, U.; Korf, H.W.; Schütz, G. Phosphorylation of CREB Ser142 Regulates Light-Induced Phase Shifts of the Circadian Clock. Neuron 2002, 34, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef] [PubMed]
- Światkiewicz, I.; Mila-Kierzenkowska, C.; Woźniak, A.; Szewczyk-Golec, K.; Nuszkiewicz, J.; Wróblewska, J.; Rajewski, P.; Eussen, S.J.P.M.; Færch, K.; Manoogian, E.N.C.; et al. Pilot Clinical Trial of Time-Restricted Eating in Patients with Metabolic Syndrome. Nutrients 2021, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hutchison, A.T.; Liu, B.; Yates, C.L.; Teong, X.T.; Wittert, G.A.; Thompson, C.H.; Nguyen, L.; Au, J.; Manoogian, E.N.C.; et al. Time-Restricted Eating Improves Glycemic Control and Dampens Energy-Consuming Pathways in Human Adipose Tissue. Nutrition 2022, 96, 111583. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Damara, M.; Misra, N.; Meziane, H.; Champy, M.F.; Chambon, P. Shifting Eating to the Circadian Rest Phase Misaligns the Peripheral Clocks with the Master SCN Clock and Leads to a Metabolic Syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef] [Green Version]
- Yamamuro, D.; Takahashi, M.; Nagashima, S.; Wakabayashi, T.; Yamazaki, H.; Takei, A.; Takei, S.; Sakai, K.; Ebihara, K.; Iwasaki, Y.; et al. Peripheral Circadian Rhythms in the Liver and White Adipose Tissue of Mice Are Attenuated by Constant Light and Restored by Time-Restricted Feeding. PLoS ONE 2020, 15, e0234439. [Google Scholar] [CrossRef]
- Hui, S.; Liu, Y.; Huang, L.; Zheng, L.; Zhou, M.; Lang, H.; Wang, X.; Yi, L.; Mi, M. Resveratrol Enhances Brown Adipose Tissue Activity and White Adipose Tissue Browning in Part by Regulating Bile Acid Metabolism via Gut Microbiota Remodeling. Int. J. Obes. 2020, 44, 1678–1690. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Justice, J.N.; De Freitas, E.C.; Kershaw, E.E.; Sparks, L.M. Adipose Tissue Quality in Aging: How Structural and Functional Aspects of Adipose Tissue Impact Skeletal Muscle Quality. Nutrients 2019, 11, 2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin Fortifies Mitochondrial Respiration in Skeletal Muscle to Promote Healthy Aging against Metabolic Challenge. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 Complex and SIR2 Alone Promote Longevity in Saccharomyces Cerevisiae by Two Different Mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.C.; Guarente, L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism That Decays with Aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian Clock Feedback Cycle through NAMPT-Mediated NAD+ Biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.M.O.; Paraíso, A.F.; De Oliveira, M.V.M.; Martins, A.M.E.; Neto, J.F.; Guimarães, A.L.S.; De Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol Attenuates Hepatic Steatosis in High-Fat Fed Mice by Decreasing Lipogenesis and Inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef] [Green Version]



| Nutrient/Dietary Components | Examples | Effect on the AT Clock | References |
|---|---|---|---|
| Fat | Whole diet | Circadian period lengthening, accompanied by an increase in food intake during the light phase and a change in AT clock gene expression in male mice, resulting in a misalignment of the circadian clock across tissues of the body. | [2,47,48] |
| Ingredients: Palmitate | Clock modulation in undifferentiated and differentiated NIH3T3 cells. | [49] | |
| Fat derivates: Free fatty acids | Natural ligands for PPARα, PPARγ and RORs, directly affecting clock gene expression | [50,51] | |
| Sugar | 30% sugar dissolved in water | Restricted access during the dark phase limits the body weight gain in rats | [52] |
| Fructose | Restricted access during the dark phase limits the AT expansion in mice | [53] | |
| Restricted access | Western diet | Restricted access during the dark phase limits the body weight gain and AT expansion in rodents, while restores the glucose tolerance and diurnal rhythms of metabolic regulators | [54,55,56] |
| Chow diet | Restricted access during the dark phase limits the body weight gain and AT expansion in rodents | [52,53] | |
| Polyphenols | Resveratrol | Increases SIRT1activity, modulates circadian rhythms of locomotor activity and body temperature, and reverse adipose tissue-specific circadian gene expression changes induced by a high fat diet | [57,58,59] |
| Procanthocyanidins (PAs) | Modulate the expression of clock-core and clock-controlled genes in mesenteric AT of healthy and obese rats, including BMAL1, Clock, Rorα, Rev-erbα, PER2 and Nampt. | [60] | |
| Epigallocatechin gallate (EGCG) | Restores BMAL1 rhythmic expression in BAT concomitant with a metabolic improvement in DIO mice | [61] | |
| Nobiletin (NOB) | Reduces fat mass and white adipocyte cell size in vivo | [62] | |
| Natural alkaloids | Caffeine | Lengthens the period of BMAL1 expression in NIH3T3 cells | [63] |
| Harmine | [64] | ||
| Lycorine | [65] | ||
| Passionflower extract | Induces high-amplitude rhythms in the expression of PER2 and CRY1 in NIH3T3 cells | [66] | |
| Endogenous biomolecules (metabolites, precursors) | Polyamines | PERiod shortening and stimulation of BMAL1 and Rev-erbα synthesis in NIH3T3 cells | [67,68] |
| Retinoic acid | Ligand for RORα. Induction of PER1 and PER2 expression in NIH3T3 cells | [69,70] | |
| Heme | Ligand for REV-ERBα | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribas-Latre, A.; Eckel-Mahan, K. Nutrients and the Circadian Clock: A Partnership Controlling Adipose Tissue Function and Health. Nutrients 2022, 14, 2084. https://doi.org/10.3390/nu14102084
Ribas-Latre A, Eckel-Mahan K. Nutrients and the Circadian Clock: A Partnership Controlling Adipose Tissue Function and Health. Nutrients. 2022; 14(10):2084. https://doi.org/10.3390/nu14102084
Chicago/Turabian StyleRibas-Latre, Aleix, and Kristin Eckel-Mahan. 2022. "Nutrients and the Circadian Clock: A Partnership Controlling Adipose Tissue Function and Health" Nutrients 14, no. 10: 2084. https://doi.org/10.3390/nu14102084
APA StyleRibas-Latre, A., & Eckel-Mahan, K. (2022). Nutrients and the Circadian Clock: A Partnership Controlling Adipose Tissue Function and Health. Nutrients, 14(10), 2084. https://doi.org/10.3390/nu14102084