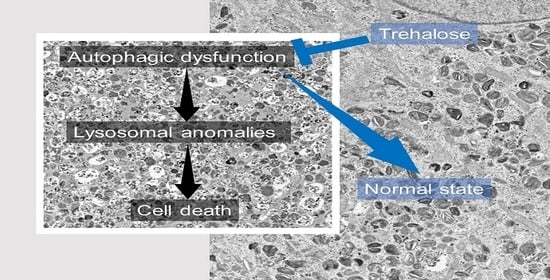
Trehalose Suppresses Lysosomal Anomalies in Supporting Cells of Oocytes and Maintains Female Fertility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Patients and Follicle Stimulation Protocol
2.3. Cumulus and MGC Preparation
2.4. Conventional In Vitro Fertilization (c-IVF) and Intracytoplasmic Sperm Injection (ICSI)
2.5. Treatment of Female Mice with Autophagy Inducer and Inhibitor
2.6. Treatment of KGN Cells with Autophagy Inducer and Inhibitor
2.7. Lysosomal Observation
2.8. Electron Microscopic Analysis
2.9. Statistical Analysis
3. Results
3.1. Characteristics and Outcomes of Patients with Infertility
3.2. Ovulation Problems Caused by Suppression of Autophagy
3.3. Lysosomal Anomalies in a Granulosa Cell-Derived Cell Line
3.4. Lysosomal Anomalies Caused by Autophagic Dysfunction in KGN Cells
3.5. Effect of Trehalose on Chloroquine-Treated KGN Cells and Female Mice
4. Discussion
4.1. Suppression of Autophagy by Chloroquine
4.2. Negative Effect of Chloroquine on Lysosomal Biogenesis
4.3. Failure of the Clearance of Problematic Lysosomes and Diseases
4.4. Therapeutic Effect of Trehalose against Human Diseases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.; Wells, D. The human oocyte and cumulus cells relationship: New insights from the cumulus cell transcriptome. Mol. Hum. Reprod. 2010, 16, 715–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, W.; Ishida, E.; Yamatoya, K.; Nakamura, A.; Miyado, M.; Miyamoto, Y.; Iwai, M.; Tatsumi, K.; Saito, T.; Saito, K.; et al. Autophagy-disrupted LC3 abundance leads to death of supporting cells of human oocytes. Biochem. Biophys. Rep. 2018, 15, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, S.; Cuervo, A.M. Chaperone-mediated autophagy: A unique way to enter the lysosome world. Trends Cell Biol. 2012, 22, 407–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukamoto, S.; Kuma, A.; Murakami, M.; Kishi, C.; Yamamoto, A.; Mizushima, N. Autophagy is essential for preimplantation development of mouse embryos. Science 2008, 321, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kuma, A.; Mizushima, N. The role of autophagy during the oocyte-to-embryo transition. Autophagy 2008, 4, 1076–1078. [Google Scholar] [CrossRef] [Green Version]
- Akino, R.; Matsui, D.; Kawahara-Miki, R.; Amita, M.; Tatsumi, K.; Ishida, E.; Kang, W.; Takada, S.; Miyado, K.; Sekizawa, A. Next-Generation Sequencing Reveals Downregulation of the Wnt Signaling Pathway in Human Dysmature Cumulus Cells as a Hallmark for Evaluating Oocyte Quality. Reprod. Med. 2020, 1, 205–215. [Google Scholar] [CrossRef]
- Saito, H.; Kaneko, T.; Takahashi, T.; Kawachiya, S.; Saito, T.; Hiroi, M. Hyaluronan in follicular fluids and fertilization of oocytes. Fertil. Steril. 2000, 74, 1148–1152. [Google Scholar] [CrossRef]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018, 9, 1–15. [Google Scholar]
- Zhao, X.-G.; Sun, R.-J.; Yang, X.-Y.; Liu, D.-Y.; Lei, D.-P.; Jin, T.; Pan, X.-L. Chloroquine-enhanced efficacy of cisplatin in the treatment of hypopharyngeal carcinoma in xenograft mice. PLoS ONE 2015, 10, e0126147. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Gao, H.; Zhou, N.; Wang, P.; Zhang, Y.; Zhang, A.; Jia, Z.; Huang, S. Trehalose attenuates renal ischemia-reperfusion injury by enhancing autophagy and inhibiting oxidative stress and inflammation. Am. J. Physiol.-Ren. Physiol. 2020, 318, F994–F1005. [Google Scholar] [CrossRef]
- Nishi, Y.; Yanase, T.; Mu, Y.; Oba, K.; Ichino, I.; Saito, M.; Nomura, M.; Mukasa, C.; Okabe, T.; Goto, K.; et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001, 142, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zheng, G.; Feng, Z.; Chen, Y.; Lou, Y.; Wang, C.; Zhang, X.; Zhang, Y.; Xu, H.; Shang, P.; et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017, 8, e3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.P.; Sousa, R.W.R.; Ferreira, J.R.O.; Militao, G.C.G.; Bezerra, D.P. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol. Res. 2021, 168, 105582. [Google Scholar] [CrossRef]
- Zeng, H.C.; Zhu, B.Q.; Wang, Y.Q.; He, Q.Z. ROS-Triggered Autophagy Is Involved in PFOS-Induced Apoptosis of Human Embryo Liver L-02 Cells. BioMed Res. Int. 2021, 2021, 6625952. [Google Scholar] [CrossRef]
- Redmann, M.; Wani, W.Y.; Volpicelli-Daley, L.; Darley-Usmar, V.; Zhang, J. Trehalose does not improve neuronal survival on exposure to alpha-synuclein pre-formed fibrils. Redox Biol. 2017, 11, 429–437. [Google Scholar] [CrossRef]
- Jeong, S.J.; Stitham, J.; Evans, T.D.; Zhang, X.; Rodriguez-Velez, A.; Yeh, Y.S.; Tao, J.; Takabatake, K.; Epelman, S.; Lodhi, I.J.; et al. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy 2021, 17, 3740–3752. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Kirshenbaum, L.A. YAP/TFEB pathway promotes autophagic cell death and hypertrophic cardiomyopathy in lysosomal storage diseases. J. Clin. Investig. 2021, 131, e146821. [Google Scholar] [CrossRef]
- Du Bois, P.; Pablo Tortola, C.; Lodka, D.; Kny, M.; Schmidt, F.; Song, K.; Schmidt, S.; Bassel-Duby, R.; Olson, E.N.; Fielitz, J. Angiotensin II Induces Skeletal Muscle Atrophy by Activating TFEB-Mediated MuRF1 Expression. Circ. Res. 2015, 117, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Khalifeh, M.; Barreto, G.E.; Sahebkar, A. Trehalose as a promising therapeutic candidate for the treatment of Parkinson’s disease. Br. J. Pharmacol. 2019, 176, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, P.; da Cunha, R.S.; Biagini, G.; Bosquetti, B.; Budag, J.; Ortiz, A.; Sanchez-Nino, M.D.; Barreto, F.C.; Stinghen, A.E.M. Chloroquine may induce endothelial injury through lysosomal dysfunction and oxidative stress. Toxicol. Appl. Pharmacol. 2021, 414, 115412. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Thorburn, A. Targeting the Lysosome for Cancer Therapy. Cancer Discov. 2017, 7, 1218–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axfors, C.; Schmitt, A.M.; Janiaud, P.; Van’t Hooft, J.; Abd-Elsalam, S.; Abdo, E.F.; Abella, B.S.; Akram, J.; Amaravadi, R.K.; Angus, D.C.; et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 2021, 12, 2349. [Google Scholar] [CrossRef]
- Settembre, C.; De Cegli, R.; Mansueto, G.; Saha, P.K.; Vetrini, F.; Visvikis, O.; Huynh, T.; Carissimo, A.; Palmer, D.; Klisch, T.J.; et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 2013, 15, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.T.; Grzeskowiak, C.L.; Fradette, J.J.; Gibson, L.A.; Rodriguez, L.B.; Creighton, C.J.; Scott, K.L.; Gibbons, D.L. TMEM106B drives lung cancer metastasis by inducing TFEB-dependent lysosome synthesis and secretion of cathepsins. Nat. Commun. 2018, 9, 2731. [Google Scholar] [CrossRef] [Green Version]
- Luzio, J.P.; Hackmann, Y.; Dieckmann, N.M.; Griffiths, G.M. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb. Perspect. Biol. 2014, 6, a016840. [Google Scholar] [CrossRef] [Green Version]
- Spaulding, H.R.; Ludwig, A.K.; Hollinger, K.; Hudson, M.B.; Selsby, J.T. PGC-1alpha overexpression increases transcription factor EB nuclear localization and lysosome abundance in dystrophin-deficient skeletal muscle. Physiol. Rep. 2020, 8, e14383. [Google Scholar] [CrossRef]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.C.; et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Zhitomirsky, B.; Yunaev, A.; Kreiserman, R.; Kaplan, A.; Stark, M.; Assaraf, Y.G. Lysosomotropic drugs activate TFEB via lysosomal membrane fluidization and consequent inhibition of mTORC1 activity. Cell Death Dis. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aits, S.; Jäättelä, M. Lysosomal cell death at a glance. J. Cell Sci. 2013, 126, 1905–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H.; Wakusawa, S.; Yano, M.; Okada, T. Genetic background of Japanese patients with adult-onset storage diseases in the liver. Hepatol. Res. 2007, 37, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Casarejos, M.; Solano, R.; Gomez, A.; Perucho, J.; De Yébenes, J.; Mena, M. The accumulation of neurotoxic proteins, induced by proteasome inhibition, is reverted by trehalose, an enhancer of autophagy, in human neuroblastoma cells. Neurochem. Int. 2011, 58, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Navarro, J.A.; Rodríguez, L.; Casarejos, M.J.; Solano, R.M.; Gómez, A.; Perucho, J.; Cuervo, A.M.; de Yébenes, J.G.; Mena, M.A. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol. Dis. 2010, 39, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Assoni, G.; Frapporti, G.; Colombo, E.; Gornati, D.; Perez-Carrion, M.D.; Polito, L.; Seneci, P.; Piccoli, G.; Arosio, D. Trehalose-based neuroprotective autophagy inducers. Bioorg. Med. Chem. Lett. 2021, 40, 127929. [Google Scholar] [CrossRef]
- Khalifeh, M.; Read, M.I.; Barreto, G.E.; Sahebkar, A. Trehalose against Alzheimer’s Disease: Insights into a Potential Therapy. Bioessays 2020, 42, 1900195. [Google Scholar] [CrossRef]
- Khalifeh, M.; Barreto, G.E.; Sahebkar, A. Therapeutic potential of trehalose in neurodegenerative diseases: The knowns and unknowns. Neural Regen. Res. 2021, 16, 2026. [Google Scholar]
- Cejka, C.; Kubinova, S.; Cejkova, J. Trehalose in ophthalmology. Histol. Histopathol. 2019, 34, 611–618. [Google Scholar]
- Takeuchi, K.; Nakazawa, M.; Ebina, Y. Effects of trehalose on VEGF-stimulated angiogenesis and myofibroblast proliferation: Implications for glaucoma filtration surgery. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6987–6993. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Nakazawa, M.; Ebina, Y.; Sato, K.; Metoki, T.; Miyagawa, Y.; Ito, T. Inhibitory effects of trehalose on fibroblast proliferation and implications for ocular surgery. Exp. Eye Res. 2010, 91, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.; Elgin, U.; Ozen, O.; Ozturk, F.G. The Efficacy and Safety of Trehalose in Primary Trabeculectomy with Mitomycin C: A Report of Early Findings. Clin. Ophthalmol. 2021, 15, 2301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, T.; Hao, Y.; Panhwar, F.; Chen, Z.; Zou, W.; Ji, D.; Chen, B.; Zhou, P.; Zhao, G. Effects of trehalose vitrification and artificial oocyte activation on the development competence of human immature oocytes. Cryobiology 2017, 74, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Robinson, C.; Danhof, H.; Knetsch, C.; Van Leeuwen, H.; Lawley, T.; Auchtung, J.; Britton, R. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature 2018, 553, 291–294. [Google Scholar] [CrossRef]
- Saund, K.; Rao, K.; Young, V.B.; Snitkin, E.S. Genetic determinants of trehalose utilization are not associated with severe Clostridium difficile infection outcome. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2020; p. ofz548. [Google Scholar]
- Eyre, D.W.; Didelot, X.; Buckley, A.M.; Freeman, J.; Moura, I.B.; Crook, D.W.; Peto, T.E.; Walker, A.S.; Wilcox, M.H.; Dingle, K.E. Clostridium difficile trehalose metabolism variants are common and not associated with adverse patient outcomes when variably present in the same lineage. EBioMedicine 2019, 43, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Yoon, J.D.; Hwang, S.-U.; Lee, J.; Kim, E.; Kim, M.; Hyun, S.-Y.; Choi, H.; Oh, D.; Jeon, Y. Exploring the mechanism of trehalose: Dual functions of PI3K/Akt and VPS34/mTOR pathways in porcine oocytes and cumulus cells. Biol. Reprod. 2022. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Ishida, E.; Amita, M.; Tatsumi, K.; Yonezawa, H.; Yohtsu, M.; Katano, D.; Onozawa, K.; Kaneko, E.; Iwasaki, W.; et al. Trehalose Suppresses Lysosomal Anomalies in Supporting Cells of Oocytes and Maintains Female Fertility. Nutrients 2022, 14, 2156. https://doi.org/10.3390/nu14102156
Kang W, Ishida E, Amita M, Tatsumi K, Yonezawa H, Yohtsu M, Katano D, Onozawa K, Kaneko E, Iwasaki W, et al. Trehalose Suppresses Lysosomal Anomalies in Supporting Cells of Oocytes and Maintains Female Fertility. Nutrients. 2022; 14(10):2156. https://doi.org/10.3390/nu14102156
Chicago/Turabian StyleKang, Woojin, Eri Ishida, Mitsuyoshi Amita, Kuniko Tatsumi, Hitomi Yonezawa, Miku Yohtsu, Daiki Katano, Kae Onozawa, Erika Kaneko, Wakako Iwasaki, and et al. 2022. "Trehalose Suppresses Lysosomal Anomalies in Supporting Cells of Oocytes and Maintains Female Fertility" Nutrients 14, no. 10: 2156. https://doi.org/10.3390/nu14102156
APA StyleKang, W., Ishida, E., Amita, M., Tatsumi, K., Yonezawa, H., Yohtsu, M., Katano, D., Onozawa, K., Kaneko, E., Iwasaki, W., Naito, N., Yamada, M., Kawano, N., Miyado, M., Sato, B., Saito, H., Saito, T., & Miyado, K. (2022). Trehalose Suppresses Lysosomal Anomalies in Supporting Cells of Oocytes and Maintains Female Fertility. Nutrients, 14(10), 2156. https://doi.org/10.3390/nu14102156