The Number of Fungiform Papillae, Taste Sensitivity and Smell Functions of Children Aged 11–15
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measuring Fungiform Papillae
2.2. Assessment of Taste Function
2.3. Olfactory Testing
2.4. Anthropometric Evaluation
2.5. Statistical Analysis
3. Results
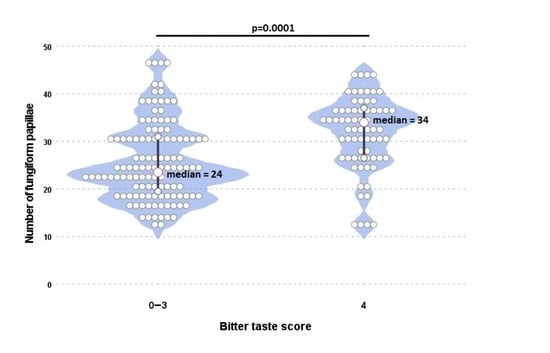
3.1. The Number of Fungiform Papillae
3.2. Taste and Smell Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breslin, P.A.; Spector, A.C. Mammalian taste perception. Curr. Biol. 2008, 18, R148–R155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Partizio, N.F. Is fat taste ready for primetime? Physiol. Behav. 2014, 136, 145–154. [Google Scholar]
- Hevezi, P.; Moyer, B.D.; Lu, M.; Gao, N.; White, E.; Echeverri, F.; Kalabat, D.; Soto, H.; Laita, B.; Li, C.; et al. Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS ONE 2009, 4, e6395. [Google Scholar] [CrossRef] [PubMed]
- Louro, T.; Simões, C.; Castelo, P.M.; Capela e Silva, F.; Luis, H.; Moreira, P.; Lamy, E. How individual variations in the perception of basic tastes and astringency relate with dietary intake and preferences for fruits and vegetables. Foods 2021, 10, 1961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-H.; Zhang, H.-Y.; Wang, X.-F.; Zhan, Y.-H.; Deng, S.-P.; Qin, Y.-M. The relationship between fungiform papillae density and detection threshold for sucrose in the young males. Chem. Senses 2009, 34, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Stamps, J.J. Chemosensory function during neurologically healthy aging. In Cognitive Changes and the Aging Brain; Heilman, K.M., Nadeau, S.E., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 68–94. [Google Scholar]
- Tepper, B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef]
- Khan, A.M.; Ali, S.; Jameela, R.V.; Muhamood, M.; Haqh, M.F. Impact of fungiform Papillae count on taste perception and different methods of taste assessment and their clinical applications. Sultan Qaboos Univ. Med. J. 2019, 19, e184–e191. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and prop bitterness depends on more than the Tas2r38 gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Bajec, M.R.; Pickering, G.J. Thermal taste, prop responsiveness, and perception of oral sensations. Physiol. Behav. 2008, 95, 581–590. [Google Scholar] [CrossRef]
- Herz, R.S.; Van Reen, E.; Gredvig-Ardito, C.A.; Carskadon, M.A. Insights into smell and taste sensitivity in normal weight and overweight-obese adolescents. Physiol. Behav. 2020, 221, 112897. [Google Scholar] [CrossRef]
- Overberg, J.; Hummel, T.; Krude, H.; Wiegand, S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch. Dis. Child. 2012, 97, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Ohla, K.; Dammann, D.; Teufel, M.; Zipfel, S.; Enck, P.; Mack, I. Changes in gustatory function and taste preference following weight loss. J. Pediatr. 2017, 182, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.A.; Beach, M.; Smith, M.C.; Chen, E.Y. Incidence of and factors associated with hypogeusia in healthy children. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 229–233. [Google Scholar] [CrossRef] [Green Version]
- De Graaf, C.; Zandstra, E.H. Sweetness intensity and pleasantness in children, adolescents, and adults. Physiol. Behav. 1999, 67, 513–520. [Google Scholar] [CrossRef]
- Malaty, J.; Malaty, I. Smell and taste disorders in primary care. Am. Fam. Physician 2013, 88, 852–859. [Google Scholar]
- Lundström, J.N.; Gordon, A.R.; Wise, P.; Frasnelli, J. Individual differences in the chemical senses: Is there a common sensitivity? Chem. Senses 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Shahbake, M.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Rapid quantitative assessment of fungiform papillae density in the human tongue. Brain Res. 2005, 1052, 196–201. [Google Scholar] [CrossRef]
- Steiner, J.E.; Glaser, D.; Hawilo, M.E.; Berridge, K.C. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neurosci. Biobehav. Rev. 2001, 25, 53–74. [Google Scholar] [CrossRef]
- Gellrich, J.; Zscheile, L.; Zickmüller, C.; Schriever, V.A. Odor identification performance in children using the ″U-Sniff″ test—Administered by an untrained person. Int. J. Pediatr. Otorhinolaryngol. 2021, 143, 110664. [Google Scholar] [CrossRef]
- Gellrich, J.; Sparing-Paschke, L.M.; Thieme, T.; Schwabe, K.; Dworschak, A.; Hummel, T.; Schriever, V.A. Normative data for olfactory threshold and odor identification in children and adolescents. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 5–9. [Google Scholar] [CrossRef]
- Herz, R.S.; Van Reen, E.; Barker, D.H.; Hilditch, C.J.; Bartz, A.L.; Carskadon, M.A. The influence of circadian timing on olfactory sensitivity. Chem. Senses 2017, 43, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kułaga, Z.; Różdżyńska, A.; Palczewska, I. Percentile charts of height, body mass and body mass index in children and adolescents in Poland—Results of the OLAF study. Stand. Med. 2010, 7, 690–700. [Google Scholar]
- Schriever, V.A.; Agosin, E.; Altundag, A.; Avni, H.; Van, H.C.; Cornejo, C.; De los Santos, G.; Fishman, G.; Fragola, C.; Guarneros, M.; et al. Development of an International Odor Identification Test for Children: The universal sniff test. J. Pediatr. 2018, 198, 265–272.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jilani, H.; Ahrens, W.; Buchecker, K.; Russo, P.; Hebestreit, A.; IDEFICS Consortium. Association between the number of fungiform papillae on the tip of the tongue and sensory taste perception in children. Food Nutr. Res. 2017, 61, 1348865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, M.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Changes in fungiform papillae density during development in humans. Chem. Senses 2013, 38, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Segovia, C.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. A quantitative study of fungiform papillae and taste pore density in adults and children. Dev. Brain Res. 2002, 138, 35–146. [Google Scholar] [CrossRef]
- Pavlidis, P.; Gouveris, C.; Kekes, G.; Maurer, J. Changes in electrogustometry thresholds, tongue tip vascularization, density and form of the fungiform papillae in smokers. Eur. Arch. Otorhinolaryngol. 2014, 271, 2325–2331. [Google Scholar] [CrossRef]
- Fischer, M.E.; Cruickshanks, K.J.; Schubert, C.R.; Pinto, A.; Klein, R.; Pankratz, N.; Pankow, J.S.; Huang, G.H. Factors related to fungiform papillae density: The beaver dam offspring study. Chem. Senses 2013, 38, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Duffy, V.B.; Hayes, J.E.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Bartoshuk, L.M. Vegetable intake in college-aged adults is explained by oral sensory phenotypes and TAS2R38 genotype. Chemosens. Percept. 2010, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Karikkineth, A.C.; Tang, E.Y.; Kuo, P.I.; Ferrucci, L.; Egan, J.M.; Chee, W. Longitudinal trajectories and determinants of human fungiform papillae density. Aging 2021, 13, 24989–25003. [Google Scholar] [CrossRef]
- Obrębowski, A.; Obrębowska-Karsznia, Z.; Gawliński, M. Smell and taste in children with simple obesity. Int. J. Pediatr. Otorhinolaryngol. 2000, 55, 191–196. [Google Scholar] [CrossRef]
- Feeney, E.L.; O’Brien, S.A.; Scannell, A.G.; Markey, A.; Gibney, E.R. Suprathreshold measures of taste perception in children-association with dietary quality and body weight. Appetite 2017, 113, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, C.; Laureati, M.; Bertoli, S.; Battezzati, A.; Pagliarini, E. Determinants of obesity in Italian adults: The role of taste sensitivity, food liking, and food neophobia. Chem. Senses 2016, 41, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite 2017, 111, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Brink, M.; IJpma, I.; Fiocco, M.; Tissing, V.J.E.; Havermans, R.C. Taste function in children: Normative values and associated factors. Pediatr. Res. 2021, 12, 1–6. [Google Scholar] [CrossRef]
- James, C.E.; Laing, D.G.; Oram, N. A comparison of the ability of 8-9-year-old children and adults to detect taste stimuli. Physiol. Behav. 1997, 62, 193–197. [Google Scholar] [CrossRef]
- Webb, J.; Bolhuis, D.P.; Cicerale, S.; Hayes, J.E.; Keast, R. The relationships between common measurements of taste function. Chemosens. Percept. 2015, 8, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, E.; Spinelli, S.; Dinnella, C.; Endrizzi, I.; Laureati, M.; Pagliarini, E.; Sinesio, F.; Gasperi, F.; Torri, L.; Aprea, E.; et al. Exploring influences on food choice in a large population sample: The Italian Taste project. Food Qual. Prefer. 2017, 59, 123–140. [Google Scholar] [CrossRef]
- Dinnella, S.C.; Monteleone, E.; Piochi, M.; Spinelli, S.; Prescott, J.; Pierguidi, L.; Gasperi, F.; Laureati, M.; Pagliarini, E.; Predieri, S.; et al. Individual variation in PROP status, fungiform papillae density, and responsiveness to taste stimuli in a large population sample. Chem. Senses 2018, 43, 697–710. [Google Scholar] [CrossRef]
- Piochi, M.; Dinnella, C.; Prescott, J.; Monteleone, E. Associations between human fungiform papillae and responsiveness to oral stimuli: Effects of individual variability, population characteristics, and methods for papillae quantification. Chem. Senses 2018, 43, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Feeney, E.; Hayes, J. Regional differences in suprathreshold intensity for bitter and umami stimuli. Chemosens. Percept. 2014, 7, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.E.; Duffy, V.B. Revisiting sugar–fat mixtures: Sweetness and creaminess vary with phenotypic markers of oral sensation. Chem. Senses 2007, 32, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essick, G.; Chopra, A.; Guest, S.; McGlone, F. Lingual tactile acuity, taste perception, and the density and diameter f fungiform papillae in female subjects. Physiol. Behav. 2003, 80, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Sullivan, B.S.; Duffy, V.B. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol Behav. 2010, 100, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delwiche, J.F.; Buletic, Z.; Breslin, P. Relationship of papillae number to bitter intensity of quinine and PROP within and between individuals. Physiol. Behav. 2001, 74, 329–337. [Google Scholar] [CrossRef]
- Gellrich, J.; Zickmüller, C.; Schriever, V.A. Assessment of olfactory function in children and adolescents: An overview. Chem. Senses 2021, 46, 27. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated sniffin’ sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, R.J.; Oaten, M.J. Sweet odours and sweet tastes are conflated in memory. Acta Psychol. 2010, 134, 105–109. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Prescott, P.; Boakes, R.A. Confusing tastes and smells: How odours can influence the perception of sweet and sour tastes. Chem. Senses 1999, 24, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Yeomans, M.R.; Prescott, J.; Gould, N.J. Acquired hedonic and sensory characteristics of odours: Influence of sweet liker and propylthiouracil taster status. Q. J. Exp. Psychol. 2009, 62, 1648–1664. [Google Scholar] [CrossRef]
- Lim, S.X.L.; Höchenberger, R.; Busch, N.A.; Bergmann, M.; Ohla, K. Associations between taste and smell sensitivity, preference and quality of life in healthy aging-the nutriact family study examinations (NFSE) Cohort. Nutrients 2022, 14, 1141. [Google Scholar] [CrossRef] [PubMed]



| All | Girls | Boys | p | |
|---|---|---|---|---|
| N (%) | 101 (100) | 46 (45.5) | 55 (54.5) | |
| Age (years) * | 13 (12–14) | 13 (12–14) | 13 (12–14) | 0.4061 a |
| Height * | 158 (149–156) | 158.5 (148–166) | 158 (150–165) | 0.5550 a |
| Weight * | 47.4 (37.7–57.6) | 27.8 (37.2–60.1) | 48.2 (38.1–57.6) | 0.5971 a |
| BMI (percentile grids) | 0.7563 b | |||
| <5 percentile | 5 (5.0) | 2 (4.3) | 3 (5.5) | |
| 5–85 percentile | 70 (69.2) | 30 (65.3) | 40 (72.7) | |
| >85 percentile | 26 (25.8) | 14 (30.4) | 12 (21.8) | |
| Total fat content (%) * | 15.4 (12.9–23.9) | 20.4 (14.8–30.3) | 12.9 (10.5–18.8) | <0.0001 a |
| All | Girls | Boys | p | |
|---|---|---|---|---|
| Number of fungiform papillae * | 27 (22–35) | 27 (22–35) | 27 (22–34) | 0.6255 a |
| PROP status | ||||
| Tasters (%) | 49 (48.5) | 19 (41.3) | 30 (54.5) | 0.1848 b |
| Nontasters (%) | 52 (51.5) | 27 (58.7) | 25 (45.5) | |
| Sensitivity to sweet taste * | 3.0 (3.0–4.0) | 3.0 (3.0–4.0) | 3.0 (3.0–4.0) | 0.3550 a |
| Sensitivity to salty taste * | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.7831 a |
| Sensitivity to sour taste * | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.6014 a |
| Sensitivity to bitter taste * | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.1321 a |
| Sensitivity to all taste * | 13 (11–14) | 14 (12–15) | 12 (9–13) | <0.0001 a |
| U-Sniff test (odour test) * | 11 (10–12) | 11 (10–12) | 11 (10–12) | 0.2455a |
| FP | PROP | All Tastes | Bitter Taste | ||
|---|---|---|---|---|---|
| FP | r | 1.000 | 0.210 | 0.300 | 0.351 |
| P | 0.035 | 0.002 | <0.001 | ||
| PROP | R | 0.210 | 1.000 | 0.374 | 0.533 |
| P | 0.035 | <0.001 | <0.001 | ||
| All taste | R | 0.300 | 0.374 | 1.000 | 0.657 |
| P | 0.002 | <0.001 | <0.001 | ||
| Bitter taste | R | 0.351 | 0.533 | 0.657 | 1.000 |
| P | <0.001 | <0.001 | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobek, G.; Jagielski, P. The Number of Fungiform Papillae, Taste Sensitivity and Smell Functions of Children Aged 11–15. Nutrients 2022, 14, 2578. https://doi.org/10.3390/nu14132578
Sobek G, Jagielski P. The Number of Fungiform Papillae, Taste Sensitivity and Smell Functions of Children Aged 11–15. Nutrients. 2022; 14(13):2578. https://doi.org/10.3390/nu14132578
Chicago/Turabian StyleSobek, Grzegorz, and Paweł Jagielski. 2022. "The Number of Fungiform Papillae, Taste Sensitivity and Smell Functions of Children Aged 11–15" Nutrients 14, no. 13: 2578. https://doi.org/10.3390/nu14132578
APA StyleSobek, G., & Jagielski, P. (2022). The Number of Fungiform Papillae, Taste Sensitivity and Smell Functions of Children Aged 11–15. Nutrients, 14(13), 2578. https://doi.org/10.3390/nu14132578