Indexing of Fatty Acids in Poultry Meat for Its Characterization in Healthy Human Nutrition: A Comprehensive Application of the Scientific Literature and New Proposals
Abstract
:1. Introduction
- -
- to come to valid considerations about the nutritional effects of different meats on human health; and
- -
- to provide a useful evaluation of the most suitable genotype and/or management practices (choice of the most appropriate feeding strategies, farming systems or post-mortem handling).
2. Materials and Methods
2.1. Animals and Diets
2.2. Sampling and Analysis
2.3. Indexes
2.3.1. Qualitative Indexes
Polyunsaturated Fatty Acid/Saturated Fatty Acid (PUFA/SFA)
n-6/n-3 Ratio
(C18:3n-3 + C20:3n-3 + C20:5n-3 + C22:5n-3 + C22:6n3)
Linoleic Acid/α-Linolenic Acid (LA/ALA) Ratio
Sum of Eicosapentaenoic Acid and Docosahexaenoic Acid (EPA + DHA%)
Unsaturation Index (UI)
(5 X % pentaenoic) + (6 X % hesaenoic)
2.3.2. Nutritional Indexes
Nutrition Value Index
Index of Atherogenicity (IA)
Index of Thrombogenicity (IT)
(3 × Σn-3 PUFA) + (Σn-3 PUFA/Σn-6 PUFA)]
Hypocholesterolemic/Hypercholesterolemic (HH) Ratio
Health-Promoting Index (HPI)
Fish Lipid Quality/Flesh Lipid Quality (FLQ)
2.3.3. Metabolic Indexes
C16:1n-7 + C18:1n-9)) × 100
+ C22:6n-3)/(C18:2n-6 + C18:3n-3+ C20:2n-6 + C20:4n-6 + C20:5n-3 + C22:5n-3
+ C22:6n-3)
2.3.4. Lipid- or Energy-Related Content Indexes
Sum of Eicosapentaenoic Acid and Docosahexaenoic Acid (EPA + DHA Quantity)
Index of Nutritional Quality
DHA daily requirement)
Proposed New Indexes
QuantiN-3 Index
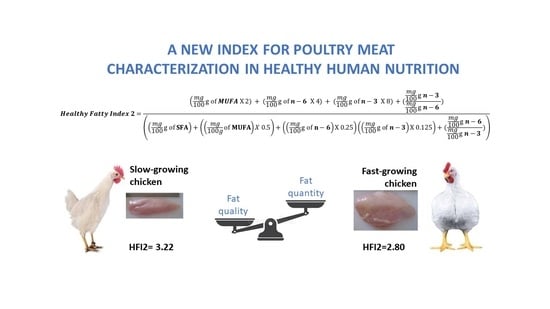
Healthy Fatty Indexes 1 and 2
8) + (mg/100 g n-3/mg/100 g n-6))/(mg/100 g of Total Lipids)
8) + (mg/100 g n-3/mg/100 g n-6))/((mg/100 g of SFA) + (mg/100 g of MUFA
× 0.5) + (mg/100 g of n-6 × 0.25) + (mg/100 g of n-3 × 0.125) + (mg/100 g n-6/mg/100 g n-3))
2.4. Statistical Analyses
3. Results and Discussion
3.1. Fatty Acid Composition (The Dataset)
3.2. Indexes
3.3. Qualitative Indexes
3.4. Nutritional Indexes
3.5. Metabolic Indexes
3.6. Lipid- or Energy Content-Related Indexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Holub, B.J. Docosahexaenoic acid (DHA) and cardiovascular disease risk factors. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Cuzzocrea, S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Fereidoon, S.; Ying, Z. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Y.; Xiao, Y.; Chen, H.; Zhao, L.; Huang, M.; Zhou, G. Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian-Australas. J. Anim. Sci. 2016, 29, 855–864. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Xie, D.; Guan, J.; Huang, X.; Xu, C.; Pan, Q.; Li, Y. Tilapia can be a Beneficial n-3 LC-PUFA Source due to Its High Biosynthetic Capacity in the Liver and Intestine. J. Agric. Food Chem. 2022, 70, 2701–2711. [Google Scholar] [CrossRef]
- Cartoni Mancinelli, A.; Mattioli, S.; Twining, C.; Dal Bosco, A.; Donoghue, A.M.; Arsi, K.; Angelucci, E.; Chiattelli, D.; Castellini, C. Poultry meat and eggs as an alternative source of n-3 long-chain polyunsaturated fatty acids for human nutrition. Nutrients 2022, 14, 1969. [Google Scholar] [CrossRef]
- Zhang, S.; Knight, T.J.; Stalder, K.J.; Goodwin, R.N.; Lonergan, S.M.; Beitz, D.C. Effects of breed, sex, and halothane genotype on fatty acid composition of pork longissimus muscle. J. Anim. Sci. 2007, 85, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Vessby, B.; Gustafsson, I.; Tengblad, S.; Boberg, M.; Andersson, A. Desaturation and elongation of fatty acids and insulin action. Ann. N. Y. Acad. Sci. 2002, 967, 183–195. [Google Scholar] [CrossRef]
- Sirri, F.; Castellini, C.; Bianchi, M.; Petracci, M.; Meluzzi, A.; Franchini, A. Effect of fast-, medium- and slow-growing strains on meat quality of chickens reared under the organic farming method. Animals 2011, 5, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Dal Bosco, A.; Mattioli, S.; Cartoni Mancinelli, A.; Cotozzolo, E.; Castellini, C. Extensive rearing systems in poultry production: The right chicken for the right farming system. A review of twenty years of scientific research in Perugia University, Italy. Animals 2021, 11, 1281. [Google Scholar] [CrossRef]
- Hosie, R.C. National Research Council. For. Regen. Ont. 2019, 91–93. [Google Scholar]
- Leeson, S.; Summers, J.D. Commercial Poultry Nutrition; Nottingham University Press: Nottingham, UK, 2009; Volume 3, ISBN 9781904761785. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Failla, S.; Buttazzoni, L.; Zilio, D.M.; Contò, M.; Renzi, G.; Castellini, C.; Amato, M.G. An index to measure the activity attitude of broilers in extensive system. Poult. Sci. 2021, 100, 101279. [Google Scholar] [CrossRef]
- Godbe, J.S. Nutritional value of muscle foods. In Muscle Foods: Meat, Poultry and Seafood Technology; Kinsman, D.M., Kotula, A.W., Breidenstein, B.C., Eds.; Chapman & Hall: London, UK; New York, NY, USA, 1994; p. 430. [Google Scholar]
- Sorenson, A.W.; Wyse, B.W.; Wittwer, A.J.; Hansen, R.G. An Index of Nutritional Quality for a balanced diet. New help for an old problem. J. Am. Diet. Assoc. 1976, 68, 236–242. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of Dietary n-3 Source on Rabbit Male Reproduction. Oxid. Med. Cell. Longev. 2019, 2019, 3279670. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.; Rebollar, P.G.; Mattioli, S.; Castellini, C. n-3 PUFA sources (precursor/products): A review of current knowledge on rabbit. Animals 2019, 9, 806. [Google Scholar] [CrossRef] [Green Version]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of dietary incorporation of linseed alone or together with tomato-red pepper mix on laying hens’ egg yolk fatty acids profile and health lipid indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Abrami, G.; Natiello, F.; Bronzi, P.; McKenzie, D.; Bolis, L.; Agradi, E. A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla anguilla). Comp. Biochem. Physiol. 1992, 101, 79–81. [Google Scholar] [CrossRef]
- Krajnovi’c-Ozretic, M.; Najdek, M.; Ozreti’c, B. Fatty acids in liver and muscle of farmed and wild sea bass (Dicentrarchus labrax L.). Comp. Biochem. Physiol. 1994, 109, 611–617. [Google Scholar] [CrossRef]
- Okada, T.; Furuhashi, N.; Kuromori, Y.; Miyashita, M.; Iwata, F.; Harada, K. Plasma palmitoleic acid content and obesity in children. Am. J. Clin. Nutr. 2005, 82, 747–750. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, S.; Cartoni Mancinelli, A.; Cotozzolo, E.; Mancini, S.; Castellini, C.; Dal Bosco, A. Comparison of an estimated index of fatty acid metabolism and liver delta6-desaturase activity in rabbit. In Proceeding of 12th World Rabbit Congress, Nantes, France, 3–5 November 2021. [Google Scholar]
- Kriketos, A.D.; Pan, D.A.; Sutton, J.R.; Hoh, J.F.; Baur, L.A.; Cooney, G.J.; Storlien, L.H. Relationships between muscle membrane lipids, fiber type, and enzyme activities in sedentary and exercised rats. Am. J. Physiol. 1995, 269, 1154–1162. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C. Fatty acid composition of meat and estimated indices of lipid metabolism in different poultry genotypes reared under organic system. Poult. Sci. 2012, 91, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Genetic variation and dietary response: Nutrigenetics/nutrigenomics. Asia Pac. J. Clin. Nutr. 2002, 11, S117–S128. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP.: College Station, TX, USA, 2015. [Google Scholar]
- Mancinelli, A.C.; Di Veroli, A.; Mattioli, S.; Cruciani, G.; Dal Bosco, A.; Castellini, C. Lipid metabolism analysis in liver of different chicken genotypes and impact on nutritionally relevant polyunsaturated fatty acids of meat. Sci. Rep. 2022, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016, 95, 2464–2471. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- FAO. Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation; FAO: Rome, Italy, 2010; Volume 91, pp. 1–189. [Google Scholar]
- Kang, M.J.; Shin, M.S.; Park, J.N.; Lee, S.S. The effects of polyunsaturated:saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br. J. Nutr. 2005, 94, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. Human requirement for N-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Mohrhauer, H.; Christiansen, K.; Gan, M.V.; Deubig, M.; Holman, R.T. Chain elongation of linoleic acid and its inhibition by other fatty acids in vitro. J. Biol. Chem. 1967, 242, 4507–4514. [Google Scholar] [CrossRef]
- Castellini, C.; Dal Bosco, A.; Mattioli, S.; Davidescu, M.; Corazzi, L.; MacChioni, L.; Rimoldi, S.; Terova, G. Activity, Expression, and Substrate Preference of the δ6-Desaturase in Slow- or Fast-Growing Rabbit Genotypes. J. Agric. Food Chem. 2016, 64, 792–800. [Google Scholar] [CrossRef]
- Harnack, K.; Andersen, G.; Somoza, V. Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutr. Metab. 2009, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Mancinelli, A.C.; Silletti, E.; Mattioli, S.; Bosco, A.D.; Sebastiani, B.; Menchetti, L.; Koot, A.; van Ruth, S.; Castellini, C. Fatty acid profile, oxidative status, and content of volatile organic compounds in raw and cooked meat of different chicken strains. Poult. Sci. 2020, 100, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Cartoni Mancinelli, A.; Menchetti, L.; Dal Bosco, A.; Madeo, L.; Guarino Amato, M.; Moscati, L.; Cotozzolo, E.; Ciarelli, C.; Angelucci, E.; et al. How the kinetic behavior of organic chickens affects productive performance and blood and meat oxidative status: A study of six poultry genotypes. Poult. Sci. 2021, 100, 101297. [Google Scholar] [CrossRef] [PubMed]
- Sinanoglou, V.J.; Strati, I.F.; Bratakos, S.M.; Proestos, C.; Zoumpoulakis, P.; Miniadis-Meimaroglou, S. On the Combined Application of Iatroscan TLC-FID and GC-FID to Identify Total, Neutral, and Polar Lipids and Their Fatty Acids Extracted from Foods. ISRN Chromatogr. 2013, 2013, 859024. [Google Scholar] [CrossRef]
- Mattioli, S.; Collodel, G.; Signorini, C.; Cotozzolo, E.; Noto, D.; Cerretani, D.; Micheli, L.; Fiaschi, A.I.; Brecchia, G.; Menchetti, L.; et al. Tissue antioxidant status and lipid peroxidation are related to dietary intake of n-3 polyunsaturated acids: A rabbit model. Antioxidants 2021, 10, 681. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Moretti, E.; Cotozzolo, E.; Perini, F.; Dal Bosco, A.; Signorini, C.; Noto, D.; Belmonte, G.; Lasagna, E.; et al. Expression of genes and localization of enzymes involved in polyunsaturated fatty acid synthesis in rabbit testis and epididymis. Sci. Rep. 2022, 12, 2637. [Google Scholar] [CrossRef]
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Wereńska, M.; Goluch, Z.; Teleszko, M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poult. Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef]
- Ghaeni, M.; Ghahfarokhi, K.N.; Zaheri, L. Fatty acids profile, atherogenic (IA) and thrombogenic (IT) health lipid indices in Leiognathusbindus and Upeneussulphureus. Journal of Marine Science. Res. Dev. 2013, 3, 1. [Google Scholar]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Laborde, F.L.; Mandell, I.B.; Tosh, J.J.; Wilton, J.W.; Buchanan-Smith, J.G. Breed effects on growth performance, carcass characteristics, fatty acid composition, and palatability attributes in finishing steers. J. Anim. Sci. 2001, 79, 355–365. [Google Scholar] [CrossRef]
- Kouba, M.; Mourot, J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie 2011, 93, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Poureslami, R.; Raes, K.; Turchini, G.M.; Huyghebaert, G.; De Smet, S. Effect of diet, sex and age on fatty acid metabolism in broiler chickens: N-3 and n-6 PUFA. Br. J. Nutr. 2010, 104, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boschetti, E.; Bordoni, A.; Meluzzi, A.; Castellini, C.; Dal Bosco, A.; Sirri, F. Fatty acid composition of chicken breast meat is dependent on genotype-related variation of FADS1 and FADS2 gene expression and desaturating activity. Animal 2016, 10, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Twining, C.W.; Brenna, J.T.; Hairston, N.G.; Flecker, A.S. Highly unsaturated fatty acids in nature: What we know and what we need to learn. Oikos 2016, 125, 749–760. [Google Scholar] [CrossRef]
- Cherian, G.; Sim, J.S. Maternal dietary α-linolenic acid (18:3n-3) alters n-3 polyunsaturated fatty acid metabolism and liver enzyme activity in hatched chicks. Poult. Sci. 2001, 80, 901–905. [Google Scholar] [CrossRef]
- Twining, C.W.; Brenna, J.T.; Lawrence, P.; Winkler, D.W.; Flecker, A.S.; Hairston, N.G. Aquatic and terrestrial resources are not nutritionally reciprocal for consumers. Funct. Ecol. 2019, 33, 2042–2052. [Google Scholar] [CrossRef]
| Starter | Finisher | |
|---|---|---|
| Ingredients | ||
| Maize | 52.0 | 46.0 |
| Full-fat soybean | 30.5 | 12.5 |
| Wheat | - | 20.0 |
| Soybean meal | 9.00 | 14.0 |
| Alfalfa meal | 2.80 | 2.80 |
| Corn gluten feed | 3.00 | 2.00 |
| Vitamin-mineral premix (1) | 1.00 | 1.00 |
| Dicalcium phosphate | 1.00 | 1.00 |
| Sodium bicarbonate | 0.50 | 0.50 |
| NaCl | 0.20 | 0.20 |
| Chemical composition | ||
| Dry matter | 90.9 | 90.8 |
| Crude protein (%) | 22.3 | 18.0 |
| Ether extract (%) | 7.95 | 4.98 |
| Crude fiber (%) | 4.67 | 4.01 |
| Ash (%) | 5.76 | 5.59 |
| NDF—Neutral Detergent Fiber | 10.7 | 10.1 |
| ADF—Acid Detergent Fiber | 5.58 | 5.06 |
| Cellulose (%) | 4.22 | 3.56 |
| ADL—Acid Detergent Liquid | 1.03 | 1.11 |
| Hemicellulose (%) | 5.16 | 5.05 |
| Metabolizable Energy (Mj/kg DM) | 12.5 | 12.9 |
| Sub- Categories | Indexes | Unit | Sign. | References |
|---|---|---|---|---|
| Qualitative |
| % | D | Many Authors |
| % | I | Simopoulus, 2008 [1]; | ||
| % | I | Undurti, 2006 [2]; | ||
| % | D | Holub, 2009 [3], Crupi and Cuzzocreas, 2022 [4]; | ||
| % | I | Shahidi and Zhong, 2010 [5]. | ||
| Nutritional |
| % | D | Chen et al., 2016 [6]; |
| % | I | Ulbricht and Southgate, 1991 [7]; | ||
| % | I | Ulbricht and Southgate, 1991 [7]; | ||
| % | D | Santos-Silva et al., 2002 [8]; | ||
| % | D | Chen et al., 2004 [9]; | ||
| % | D | Xie et al., 2022 [10]. | ||
| Metabolic |
| % | - | Zhang et al., 2007 [12]]; |
| % | - | Zhang et al., 2007 [12]; | ||
| % | - | Vessby et al., 2002 [13]; | ||
| % | - | Vessby et al., 2002 [13]; | ||
| % | - | Sirri et al., 2011 [14]; | ||
| % | - | Failla et al., 2021 [19]. | ||
| Lipid- or energy-related content indexes |
| mg/100 g | D | Godbe, 1994 [20]; |
| mg/100 g | D | Sorenson et al., 1976 [21]; | ||
| mg/100 g | D | Present paper; | ||
| mg/100 g | D | Present paper; | ||
| mg/100 g | D | Present paper. |
| SG | FG | SEM | SG | FG | SEM | |
|---|---|---|---|---|---|---|
| Lipids (g/100 g meat) | 0.25 B | 1.45 A | 0.13 | |||
| % of total FA | mg/100 g meat | |||||
| C12:0 | 0.20 B | 0.62 A | 0.09 | 0.43 B | 7.41 A | 1.03 |
| C14:0 | 0.71 B | 1.04 A | 0.21 | 1.54 B | 12.42 A | 2.21 |
| C16:0 | 27.98 b | 30.01 a | 2.03 | 60.53 B | 358.45 A | 125.14 |
| C18:0 | 12.02 a | 11.00 b | 1.61 | 30.33 B | 131.39 A | 20.14 |
| Others | 2.49 | 2.07 | 0.20 | 7.55 B | 24.72 A | 3.56 |
| Total SFA | 43.40 a | 44.74 b | 2.11 | 100.38 B | 534.39 A | 156.48 |
| C14:1n-6 | 0.15 | 0.11 | 0.04 | 0.32 B | 1.31 A | 0.24 |
| C16:1n-7 | 0.61 A | 1.39 B | 0.19 | 1.32 B | 16.60 A | 3.14 |
| C18:1n-9 | 19.50 A | 23.04 B | 2.31 | 40.02 B | 275.20 A | 10.41 |
| Others | 0.26 | 0.17 | 0.91 | 0.56 a | 2.03 b | 0.48 |
| Total MUFA | 20.52 A | 23.94 B | 1.75 | 42.23 B | 293.95 A | 23.47 |
| C18:2n-6 | 18.09 B | 20.70 A | 2.18 | 39.14 B | 247.25 A | 25.36 |
| C20:2n-6 | 0.68 a | 0.20 b | 0.16 | 1.47 b | 2.39 a | 0.23 |
| C20:3n-6 | 0.17 | 0.16 | 0.03 | 0.37 B | 1.91 A | 0.47 |
| C20:4n-6 | 10.60 a | 8.03 b | 1.36 | 22.93 B | 95.91 A | 15.98 |
| Total n-6 | 29.54 | 29.09 | 2.47 | 63.91 B | 347.46 A | 36.98 |
| C18:3n-3 | 2.03 a | 0.98 b | 0.74 | 2.23 B | 11.71 A | 4.12 |
| C20:3n-3 | 0.04 | 0.03 | 0.01 | 0.09 B | 0.36 A | 0.13 |
| C20:5n-3 | 0.68 A | 0.09 B | 0.27 | 0.39 B | 1.07 A | 0.32 |
| C21:5n-3 | 0.87 A | 0.03 B | 0.47 | 1.88 A | 0.36 B | 0.24 |
| C22:5n-3 | 1.37 A | 0.92 B | 0.25 | 2.96 B | 10.99 A | 1.79 |
| C22:6n-3 | 1.05 A | 0.18 B | 0.29 | 2.26 | 2.15 | 0.14 |
| Total n-3 | 4.54 A | 2.23 B | 1.75 | 9.81 B | 26.64 A | 3.49 |
| Total PUFA | 35.58 A | 31.32 B | 3.04 | 73.72 B | 374.10 A | 98.85 |
| Indexes | SG | FG | SEM |
|---|---|---|---|
| Qualitative | |||
| Polyunsaturated/Saturated Fatty Acids | 0.81 a | 0.70 b | 0.06 |
| n-6/n-3 Fatty Acids Ratio | 4.89 B | 13.04 A | 1.89 |
| Linoleic Acid/α-Linolenic Acid | 8.91 B | 21.12 A | 2.15 |
| Sum of EPA and DHA | 1.73 A | 0.27 B | 0.23 |
| Unsaturation Index | 128.05 A | 107.75 B | 5.36 |
| Nutritional | |||
| Nutritional Value Index | 1.11 | 1.14 | 0.14 |
| Index of Atherogenicity | 0.55 b | 0.63 a | 0.07 |
| Index of Thrombogenicity | 0.94 b | 1.24 a | 0.10 |
| Hypocholesterolemic/Hypercholesterolemic | 1.91 A | 1.21 B | 0.12 |
| Health-Promoting Index | 1.81 a | 1.59 b | 0.17 |
| Fish Lipid Quality/Flesh Lipid Quality | 1.73 A | 0.27 B | 0.12 |
| Metabolic | |||
| Elongase Index | 0.47 a | b | 0.09 |
| Thioesterase Index | 42.7 A | 30.9 B | 4.23 |
| Δ9-Desaturase (18) | 58.0 B | 68.7 A | 3.65 |
| Δ9-Desaturase (16 + 18) | 31.40 b | 37.51 a | 6.32 |
| Δ5/Δ6-Desaturase | 52.48 A | 23.55 B | 4.92 |
| Activity index | 1.97 A | 1.28 B | 0.12 |
| Lipid- or energy-related content indexes | |||
| Sum of EPA and DHA (mg/100 g meat) | 3.72 a | 3.22 b | 0.15 |
| Index of Nutritional Quality | 9.42 A | 7.29 B | 0.74 |
| Quanti n-3 Index | 53.23 A | 18.37 B | 4.05 |
| Healthy Fatty Index 1 | 1.80 a | 1.49 b | 0.08 |
| Healthy Fatty Index 2 | 3.22 A | 2.80 B | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Bosco, A.; Cartoni Mancinelli, A.; Vaudo, G.; Cavallo, M.; Castellini, C.; Mattioli, S. Indexing of Fatty Acids in Poultry Meat for Its Characterization in Healthy Human Nutrition: A Comprehensive Application of the Scientific Literature and New Proposals. Nutrients 2022, 14, 3110. https://doi.org/10.3390/nu14153110
Dal Bosco A, Cartoni Mancinelli A, Vaudo G, Cavallo M, Castellini C, Mattioli S. Indexing of Fatty Acids in Poultry Meat for Its Characterization in Healthy Human Nutrition: A Comprehensive Application of the Scientific Literature and New Proposals. Nutrients. 2022; 14(15):3110. https://doi.org/10.3390/nu14153110
Chicago/Turabian StyleDal Bosco, Alessandro, Alice Cartoni Mancinelli, Gaetano Vaudo, Massimiliano Cavallo, Cesare Castellini, and Simona Mattioli. 2022. "Indexing of Fatty Acids in Poultry Meat for Its Characterization in Healthy Human Nutrition: A Comprehensive Application of the Scientific Literature and New Proposals" Nutrients 14, no. 15: 3110. https://doi.org/10.3390/nu14153110
APA StyleDal Bosco, A., Cartoni Mancinelli, A., Vaudo, G., Cavallo, M., Castellini, C., & Mattioli, S. (2022). Indexing of Fatty Acids in Poultry Meat for Its Characterization in Healthy Human Nutrition: A Comprehensive Application of the Scientific Literature and New Proposals. Nutrients, 14(15), 3110. https://doi.org/10.3390/nu14153110