Familial Mediterranean Fever and Diet: A Narrative Review of the Scientific Literature
Abstract
:1. Introduction
2. Materials and Methods
[(“familial Mediterranean fever” OR “MEFV” OR “FMF” OR “Mediterranean fever” OR “periodic fever syndrome”) AND (diet* OR food OR nutrition OR regimen OR habit OR wheat OR gluten OR milk OR nutrient OR egg OR fat)].
Inclusion/Exclusion Criteria
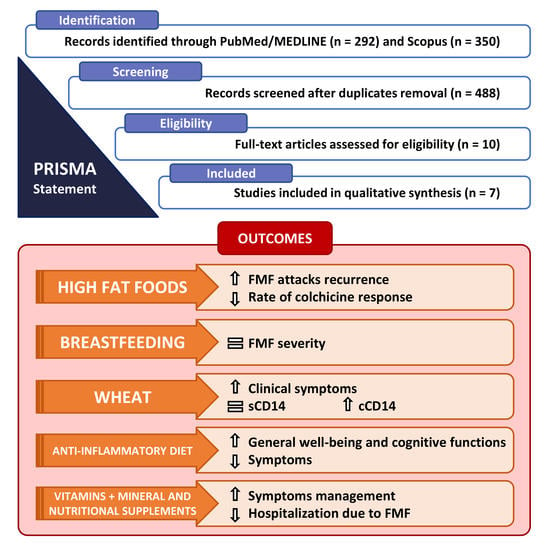
3. Results
3.1. Literature Search
3.2. Characteristics of the Included Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alghamdi, M. Familial Mediterranean fever, review of the literature. Clin. Rheumatol. 2017, 36, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 1997, 90, 797–807. [CrossRef]
- Ben-Chetrit, E.; Touitou, I. Familial Mediterranean Fever in the World. Arthritis Care Res. 2009, 61, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Izumi, Y.; Jiuchi, Y.; Iwanaga, N.; Kawahara, C.; Agematsu, K.; Yachie, A.; Masumoto, J.; Fujikawa, K.; Yamasaki, S.; et al. Familial Mediterranean fever is no longer a rare disease in Japan. Arthritis Res. Ther. 2016, 18, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onen, F.; Sumer, H.; Turkay, S.; Akyurek, O.; Tunca, M.; Ozdogan, H. Increased frequency of familial Mediterranean fever in Central Anatolia, Turkey. Clin. Exp. Rheumatol. 2004, 22 (Suppl 34), S31–S33. [Google Scholar] [PubMed]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Pathak, S.; McDermott, M.F.; Savic, S. Autoinflammatory diseases: Update on classification diagnosis and management. J. Clin. Pathol. 2017, 70, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ozen, S.; Kone-Paut, I.; Gül, A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin. Arthritis Rheum. 2017, 47, 115–120. [Google Scholar] [CrossRef]
- Zarouk, W.A.; El-Bassyouni, H.T.; Ramadan, A.; Fayez, A.; Esmaiel, N.N.; Foda, B.M.; Kobiesy, M.M.; Zekry, M.E.; Lotfy, R.S.; Shehata, G.M. Screening of the most common MEFV mutations in a large cohort of Egyptian patients with Familial Mediterranean fever. Gene Rep. 2018, 11, 23–28. [Google Scholar] [CrossRef]
- Salehzadeh, F.; Mohammadikebar, Y.; Haghi, R.; Asl, S.; Enteshary, A. Familial Mediterranean Fever Gene Mutations and Gout as an Auto-Inflammatory Arthropathy. Med Arch. 2019, 73, 55–57. [Google Scholar] [CrossRef]
- Bashardoust, B. Familial Mediterranean fever; diagnosis, treatment, and complications. J. Nephropharmacol. 2015, 4, 5–8. [Google Scholar]
- Demir, A.; Akyüz, F.; Gokturk, S.; Evirgen, S.; Akyüz, U.; Örmeci, A.; Soyer, O.M.; Karaca, C.; Demir, K.; Gundogdu, G.; et al. Small bowel mucosal damage in familial Mediterranean fever: Results of capsule endoscopy screening. Scand. J. Gastroenterol. 2014, 49, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Kasifoglu, T.; Bilge, S.Y.; Sari, I.; Solmaz, D.; Senel, S.; Emmungil, H.; Kilic, L.; Oner, S.Y.; Yildiz, F.; Yilmaz, S.; et al. Amyloidosis and its related factors in Turkish patients with familial Mediterranean fever: A multicentre study. Rheumatology 2014, 53, 741–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guler, T.; Garip, Y.; Dortbas, F.; Dogan, Y.P. Quality of life in Turkish patients with Familial Mediterranean Fever: Association with fatigue, psychological status, disease severity and other clinical parameters. Egypt. Rheumatol. 2018, 40, 117–121. [Google Scholar] [CrossRef]
- Ozcan, A.V. Cardiac tamponade caused by Familial Mediterranean fever: A case report. Turk. J. Thorac. Cardiovasc. Surg. 2012, 20, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Migita, K.; Abiru, S.; Sasaki, O.; Miyashita, T.; Izumi, Y.; Nishino, A.; Jiuchi, Y.; Kawakami, A.; Yasunami, M. Coexistence of familial Mediterranean fever and rheumatoid arthritis. Mod. Rheumatol. 2014, 24, 212–216. [Google Scholar] [CrossRef]
- Zemer, D.; Pras, M.; Sohar, E.; Gafni, J. Colchicine in Familial Mediterranean Fever. N. Engl. J. Med. 1976, 294, 170–171. [Google Scholar] [CrossRef]
- Hentgen, V.; Grateau, G.; Kone-Paut, I.; Livneh, A.; Padeh, S.; Rozenbaum, M.; Amselem, S.; Gershoni-Baruch, R.; Touitou, I.; Ben-Chetrit, E. Evidence-based recommendations for the practical management of Familial Mediterranean Fever. Semin. Arthritis Rheum. 2013, 43, 387–391. [Google Scholar] [CrossRef]
- Sari, I.; Birlik, M.; Kasifoglu, T. Familial Mediterranean fever: An updated review. Eur. J. Rheumatol. 2014, 1, 21–33. [Google Scholar] [CrossRef]
- Shohat, M.; Halpern, G.J. Familial Mediterranean fever—A review. Genet. Med. 2011, 13, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, A.D.; Peters, R.S. Familial mediterranean fever in armenians. Analysis of 100 cases. Medicine 1974, 53, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Makay, B.; Unsal, E. Does breast-feeding affect severity of familial Mediterranean fever? Clin. Rheumatol. 2009, 28, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Yenokyan, G.; Armenian, H.K. Triggers for Attacks in Familial Mediterranean Fever: Application of the Case-Crossover Design. Am. J. Epidemiol. 2012, 175, 1054–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekinci, R.M.K.; Balcı, S.; Bisgin, A.; Cetin, F.T.; Tumgor, G. The contribution of diet preference to the disease course in children with familial Mediterranean fever: A cross-sectional study. Reumatologia 2020, 58, 81–86. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Soresi, M.; Fayer, F.; Di Liberto, D.; Monguzzi, E.; Pizzo, M.L.; La Blasca, F.; Geraci, G.; Pecoraro, A.; et al. Wheat Consumption Leads to Immune Activation and Symptom Worsening in Patients with Familial Mediterranean Fever: A Pilot Randomized Trial. Nutrients 2020, 12, 1127. [Google Scholar] [CrossRef] [Green Version]
- Kazem, Y.; Zarouk, W.A.; Hamed, K.; Tosson, A.; Essa, H.A.; El-Bassyouni, H.T. The Effect of Anti-inflammatory Diet and Vitamin D Supplementation on the Amelioration of the Clinical Status and Cognitive functions of Familial Mediterranean, Fever Patients. Kobe J. Med. Sci. 2021, 66, E159–E165. [Google Scholar]
- Şentürk, S.; Arslan, D.E.; Çetinkaya, A. Association of complementary and integrative therapy use and symptoms among Turkish patients with familial Mediterranean fever. J. Integr. Med. 2021, 19, 340–346. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Masson, C.J.; Mensink, R.P. Exchanging Saturated Fatty Acids for (n-6) Polyunsaturated Fatty Acids in a Mixed Meal May Decrease Postprandial Lipemia and Markers of Inflammation and Endothelial Activity in Overweight Men. J. Nutr. 2011, 141, 816–821. [Google Scholar] [CrossRef]
- Rambod, M.; Nazarinia, M.; Raieskarimian, F. The impact of dietary habits on the pathogenesis of rheumatoid arthritis: A case-control study. Clin. Rheumatol. 2018, 37, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.-M.; Nita, I.E.; Olteanu, R.; Constantin, T.; Bucur, S.; Matei, C.; Raducan, A. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis (Review). Exp. Ther. Med. 2019, 17, 1085–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scrivo, R.; Perricone, C.; Altobelli, A.; Castellani, C.; Tinti, L.; Conti, F.; Valesini, G. Dietary Habits Bursting into the Complex Pathogenesis of Autoimmune Diseases: The Emerging Role of Salt from Experimental and Clinical Studies. Nutrients 2019, 11, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celiberto, L.; Graef, F.; Healey, G.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018, 155, 36–52. [Google Scholar] [CrossRef]
- Calder, P.C. Immunonutrition. BMJ 2003, 327, 117–118. [Google Scholar] [CrossRef]
- Grimble, R.F. Basics in clinical nutrition: Immunonutrition—Nutrients which influence immunity: Effect and mechanism of action. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, e10–e13. [Google Scholar] [CrossRef] [Green Version]
- Deshayes, S.; Fellahi, S.; Bastard, J.-P.; Launay, J.-M.; Callebert, J.; Fraisse, T.; Buob, D.; Boffa, J.-J.; Giurgea, I.; Dupont, C.; et al. Specific changes in faecal microbiota are associated with familial Mediterranean fever. Ann. Rheum. Dis. 2019, 78, 1398–1404. [Google Scholar] [CrossRef]
- Khachatryan, Z.A.; Ktsoyan, Z.A.; Manukyan, G.P.; Kelly, D.; Ghazaryan, K.A.; Aminov, R.I. Predominant Role of Host Genetics in Controlling the Composition of Gut Microbiota. PLoS ONE 2008, 3, e3064. [Google Scholar] [CrossRef] [Green Version]
- Ktsoyan, Z.A.; Beloborodova, N.V.; Sedrakyan, A.M.; Osipov, G.A.; Khachatryan, Z.A.; Kelly, D.; Manukyan, G.P.; Arakelova, K.A.; Hovhannisyan, A.I.; Olenin, A.Y.; et al. Profiles of Microbial Fatty Acids in the Human Metabolome are Disease-Specific. Front. Microbiol. 2011, 1, 148. [Google Scholar] [CrossRef] [Green Version]
- Di Ciaula, A.; Stella, A.; Bonfrate, L.; Wang, D.Q.H.; Portincasa, P. Gut Microbiota between Environment and Genetic Background in Familial Mediterranean Fever (FMF). Genes 2020, 11, 1041. [Google Scholar] [CrossRef]
- Demirtürk, L.; Özel, A.; Cekem, K.; Yazgan, Y.; Gultepe, M. Co-existence of Helicobacter pylori infection in patients with Familial Mediterranean Fever (FMF) and the effect of Helicobacter pylori on the frequency and severity of FMF attacks. Dig. Liver Dis. 2005, 37, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ozel, A.M.; Demirturk, L.; Aydogdu, A.; Gultepe, M.; Yazgan, Y.; Imirzalioglu, N.; Gurbuz, A.K.; Narin, Y. Effect of Helicobacter pylori infection and eradication therapy on interleukin-6 levels in patients with Familial Mediterranean Fever. Int. J. Clin. Pr. 2008, 62, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, E.; Sicignano, L.L.; La Regina, M.; Nucera, G.; Patisso, I.; Cerrito, L.; Montalto, M.; Gasbarrini, A.; Manna, R. Small Intestinal Bacterial Overgrowth Affects the Responsiveness to Colchicine in Familial Mediterranean Fever. Mediat. Inflamm. 2017, 2017, 7461426. [Google Scholar] [CrossRef] [PubMed]
- Mellinkoff, S.M. A Dietary Treatment for Familial Mediterranean Fever. Arch. Intern. Med. 1961, 108, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Khachadurian, A.K.; Armenian, H.K. The management of familial paroxysmal polyserositis (familial Medi-terranean fever). Experience with low-fat diets and Clofibrate. Le Journal medical libanais. Leban. Med. J. 1972, 25, 495–502. [Google Scholar]
- Reimann, H.A. The low-fat diet for periodic peritonitis. Am. J. Gastroenterol. 1962, 38, 85–90. [Google Scholar]
- Sohar, E.; Gafni, J.; Chaimow, M.; Prass, M.; Heller, H. Low-Fat Diet in Familial Mediterranean Fever: A therapeutic trial. Arch. Intern. Med. 1962, 110, 150–154. [Google Scholar] [CrossRef]
- Piram, M.; Koné-Paut, I.; Lachmann, H.; Frenkel, J.; Ozen, S.; Kuemmerle-Deschner, J.; Stojanov, S.; Simon, A.; Finetti, M.; Sormani, M.P.; et al. Validation of the Auto-Inflammatory Diseases Activity Index (AIDAI) for hereditary recurrent fever syndromes. Ann. Rheum. Dis. 2014, 73, 2168–2173. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Tufan, A.; Lachmann, H.J. Familial Mediterranean fever, from pathogenesis to treatment: A contemporary review. Turk. J. Med. Sci. 2020, 50, 1591–1610. [Google Scholar] [CrossRef]
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Abbate, R.; Gensini, G.F.; Benedettelli, S.; Casini, A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: A double-blinded randomised dietary intervention trial. Br. J. Nutr. 2014, 111, 1992–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Giangrandi, I.; Colombini, B.; Gori, A.M.; Fiorillo, C.; Becatti, M.; Casini, A.; Benedettelli, S.; et al. A Khorasan Wheat-Based Replacement Diet Improves Risk Profile of Patients with Nonalcoholic Fatty Liver Disease (NAFLD): A Randomized Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisslitz, S.; Longin, C.F.H.; Koehler, P.; Scherf, K.A. Comparative quantitative LC–MS/MS analysis of 13 amylase/trypsin inhibitors in ancient and modern Triticum species. Sci. Rep. 2020, 10, 14570. [Google Scholar] [CrossRef] [PubMed]
- Spisni, E.; Imbesi, V.; Giovanardi, E.; Petrocelli, G.; Alvisi, P.; Valerii, M.C. Differential Physiological Responses Elicited by Ancient and Heritage Wheat Cultivars Compared to Modern Ones. Nutrients 2019, 11, 2879. [Google Scholar] [CrossRef] [Green Version]

| Reference Article [Reference No] | Publication Year | Year(s) of Study | Country | Ethnicity | Study Design | Primary Objective of the Study | No. of Participants | Outcome | Food Considered | Findings and Interpretations |
|---|---|---|---|---|---|---|---|---|---|---|
| Schwabe, A.D. [21] | 1974 | 1960–1972 | USA | Armenian | Retrospective study | To review the clinical manifestations, complications, and prognosis in 100 Armenians with FMF followed for 2–12 years. To assess differences in FMF attack recurrence in a subgroup of 46 patients undergoing the 20-g-of-fat daily diet. | 100 | FMF attack recurrence | Fatty foods (sausage, pork, eggs, and ice-cream); alcohol | No conclusions can be drawn from this limited study. Adherence to the 20-g-of-fat daily diet represented the major problem in these patients since only 18 out of 46 patients fully followed the above-mentioned diet for 2 years, without statistically significant results. |
| Makay, B. [22] | 2009 | 2009 | Turkey | Turkish | Retrospective study | To investigate whether being breastfed and duration of breastfeeding has an impact on the phenotypic expression of FMF | 81 | FMF severity | Breastfeeding, formula feeding, cow’s milk feeding, complementary feeding | Breastfeeding is not an exogenous factor having an impact on FMF disease severity. Further collaborative studies on large series from different geographic regions investigating the effect of breastfeeding on severity of FMF are required. |
| Yenokyan, G. [23] | 2012 | 2007–2008 | Armenia | Armenian | Case-crossover study | To estimate if stressful events, like a high-fat diet, represent a trigger for FMF attacks in a restricted exposure window | 167 | Time between consumption of high-fat foods and FMF attacks | High-fat diet (beef, pork, other lunch meat, butter, mayonnaise, eggs, cheese, milk, popcorn, French fries, sour cream, yogurt, ice cream, pastry) | Statistically significant negative relation between consumption of high-fat-containing food items and the likelihood of developing FMF attacks. |
| Ekinci, R. [24] | 2020 | 2019 | Turkey | Turkish | Retrospective cross-sectional study | To assess diet behaviors and self-efficacy in children with FMF and the relation with symptoms, attack frequency, and treatment outcomes | 74 | Distribution of MEFV mutations, CDSS and DBS scores pooled and relation with foods | High-fat and high-salt foods | Statistically significant higher rate of complete colchicine response in patients with a preference for less salty or fatty meals. The symptoms and laboratory results did not differ between patients grouped according to their dietary self-efficacy and behaviors. |
| Carroccio, A. [25] | 2020 | 2015–2017 | Italy | Italian | Case-crossover study | (1) To determine if a 2-week double-blind placebo-controlled (DPBC) crossover wheat vs. rice challenge exacerbates clinical manifestations of FMF; (2) To evaluate the innate response of non-celiac wheat sensitivity (NCWS)/FMF patients who underwent the DPBC challenge | 6 | (1) Clinical symptoms, by an FMF-specific AIDAI (Auto-Inflammatory Diseases Activity Index) score; (2) Serum soluble CD14 (sCD14), C-reactive protein (CRP), and serum amyloid A (SSA); (3) Circulating CD14+ monocytes expressing IL-1β and TNF-α | Wheat | The AIDAI score significantly increased in FMF patients during DBPC with wheat, but not with rice (19 ± 6.3 vs. 7 ± 1.6; p = 0.028). sCD14 values did not differ in FMF patients before and after the challenge but were higher in FMF patients than in healthy controls (median values 11357 vs. 8710 pg/mL; p = 0.002). The percentage of circulating CD14+/IL-1β+ and CD14+/TNF-α+ monocytes increased significantly after DBPC with wheat vs. baseline or rice challenge. |
| Kazem, Y. [26] | 2020 | 2017–2018 | Egypt | Egyptian | Retrospective and cross-sectional study (before/after) | To highlight the effect of an anti-inflammatory diet, containing vitamin D, curcumin and flaxseed supplementation, on the clinical presentation, general well-being and cognitive functions of a group of FMF patients | 73 | FMF attack recurrence, subjective well being | Anti-inflammatory diet (rich in fresh vegetables and fruits, low in saturated and unsaturated fats and carbohydrates, low in food additives, sugar, fast foods and processed foods) + dietary supplementation with vitamin D, curcumin and flaxseeds | The anti-inflammatory diet, containing vitamin D, curcumin and flaxseed supplementation, ameliorated the clinical presentation, general well-being and cognitive functions of FMF patients |
| Şentürk, S. [27] | 2021 | 2018–2019 | Turkey | Turkish | Retrospective and cross-sectional study | To evaluate correlations between the use of complementary and integrative therapies (CIT) and the symptoms of Turkish patients with FMF. The study sought to answer the following questions: (1) What is the frequency of CIT use in disease management in Turkish patients with FMF? (2) What CIT modalities are used by Turkish patients with FMF? (3) Is there a relationship between CIT use and symptoms in Turkish patients with FMF? | 1119 | FMF-related hospitalization | Mineral supplements and vitamins (calcium, iron, zinc, and vitamin B12, C, D and E); nutritional supplements (fish oil, honey, and ginseng pills) | Statistically significant relationship between having five or more health check-ups per year and CIT use. Individuals who are very aware of their own health seem to be more likely to use CIT for symptom management, suggesting that individuals with FMF effectively benefit from health care. At the same time, a statistically significant negative relationship was found between hospitalization due to FMF and CIT use. This result suggests that individuals with FMF used CIT methods in an uncontrolled manner. However, as the questionnaire did not ask how CIT methods were used, this relationship may be more complicated. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansueto, P.; Seidita, A.; Chiavetta, M.; Genovese, D.; Giuliano, A.; Priano, W.; Carroccio, A.; Casuccio, A.; Amodio, E. Familial Mediterranean Fever and Diet: A Narrative Review of the Scientific Literature. Nutrients 2022, 14, 3216. https://doi.org/10.3390/nu14153216
Mansueto P, Seidita A, Chiavetta M, Genovese D, Giuliano A, Priano W, Carroccio A, Casuccio A, Amodio E. Familial Mediterranean Fever and Diet: A Narrative Review of the Scientific Literature. Nutrients. 2022; 14(15):3216. https://doi.org/10.3390/nu14153216
Chicago/Turabian StyleMansueto, Pasquale, Aurelio Seidita, Marta Chiavetta, Dario Genovese, Alessandra Giuliano, Walter Priano, Antonio Carroccio, Alessandra Casuccio, and Emanuele Amodio. 2022. "Familial Mediterranean Fever and Diet: A Narrative Review of the Scientific Literature" Nutrients 14, no. 15: 3216. https://doi.org/10.3390/nu14153216
APA StyleMansueto, P., Seidita, A., Chiavetta, M., Genovese, D., Giuliano, A., Priano, W., Carroccio, A., Casuccio, A., & Amodio, E. (2022). Familial Mediterranean Fever and Diet: A Narrative Review of the Scientific Literature. Nutrients, 14(15), 3216. https://doi.org/10.3390/nu14153216