Highland Barley Polyphenol Delayed the In Vitro Digestibility of Starch and Amylose by Modifying Their Structural Properties
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of Highland Barley Polyphenol (HBP)
2.2.2. Preparation of Polyphenol-Starch Complexes
2.3. Total Phenolic Content, Total Flavonoid Content and Total Antioxidant Activity
2.4. Binding Ability
2.5. Scanning Electron Microscope (SEM)
2.6. Fourier Transforms Infrared (FT-IR) Spectroscopy
2.7. X-ray Diffraction (XRD)
2.8. Differential Scanning Calorimeter (DSC)
2.9. In Vitro Gastrointestinal Digestion
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Compounds and Antioxidant Activity
3.2. Binding Ability of HBP-Starch Complexes
3.3. Complexation between HBP and Starch
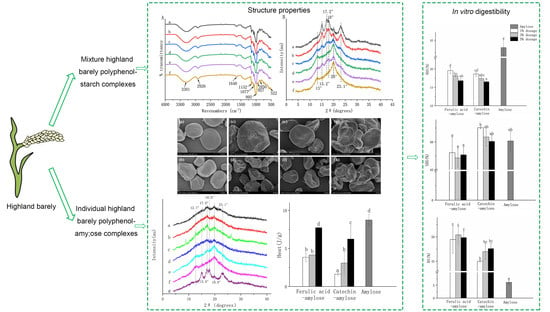
3.4. Microstructure of HBP-Starch Complexes
3.5. In Vitro Digestibility of HBP-Starch Complexes
3.6. Characterization of Amylose Compound with Main Monomer Phenol
3.6.1. In Vitro Digestibility of Amylose Compound with Main Monomer Phenol
3.6.2. Crystallinity and Thermal Properties of Amylose Compound with Main Monomer Phenol in Highland Barley
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, T.; Wang, K.; Zhao, L.; Chen, Y.; Zhou, W.; Liu, F.; Hu, Z. Interaction with longan seed polyphenols affects the structure and digestion properties of maize starch. Carbohydr. Polym. 2021, 256, 117537. [Google Scholar] [CrossRef] [PubMed]
- Nadia, J.; Bronlund, J.; Singh, R.; Singh, H.; Bornhorst, G. Structural breakdown of starch-based foods during gastric digestion and its link to glycemic response: In vivo and in vitro considerations. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2660–2698. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ogawa, Y.; Shi, J.; Chen, S.; Zhang, H.; Liu, D.; Ye, X. The microstructure of starchy food modulates its digestibility. Crit. Rev. Food Sci. Nutr. 2019, 59, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, S.; Hwang, J.; Benndorf, R.; Beemelmanns, C.; Chung, S.; Kim, K.; Fridamycin, A. A microbial natural product, stimulates glucose uptake without inducing adipogenesis. Nutrients 2019, 11, 765. [Google Scholar] [CrossRef]
- Nanjan, M.; Mohammed, M.; Prashantha, K.; Chandrasekar, M. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg. Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, B.; Yan, Y.; Liang, J.; Guan, X. Bound polyphenols from red quinoa prevailed over free polyphenols in reducing postprandial blood glucose rises by inhibiting α-Glucosidase activity and starch digestion. Nutrients 2022, 14, 728. [Google Scholar] [CrossRef]
- Amoako, D.; Awika, J. Resistant starch formation through intrahelical V-complexes between polymeric proanthocyanidins and amylose. Food Chem. 2019, 285, 326–333. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trend Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Kong, X.; Yang, W.; Yin, X.; Xu, E.; Chen, S.; Liu, D.; Ye, X. Physicochemical and digestibility characterisation of maize starch-caffeic acid complexes. LWT—Food Sci. Technol. 2020, 121, 108857. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trend Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, L.; Wu, X.; Li, W.; Wu, T.; Zhang, P. Impact of germination pretreatment on the polyphenol profile, antioxidant activities, and physicochemical properties of three color cultivars of highland barley. J. Cereal Sci. 2021, 97, 103152. [Google Scholar] [CrossRef]
- Karunaratne, R.; Zhu, F. Physicochemical interactions of maize starch with ferulic acid. Food Chem. 2016, 199, 372–379. [Google Scholar] [CrossRef]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Li, C.; Meng, Q.; Meng, Y.; Ying, J.; Bai, S.; Shen, Q.; Xue, Y. Beneficial effects of partly milled highland barley on the prevention of high-fat diet-induced glycometabolic disorder and the modulation of gut microbiota in mice. Nutrients 2022, 14, 762. [Google Scholar] [CrossRef]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trend Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Anoma, C.; Fereidoon, S. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 2011, 3, 144–158. [Google Scholar]
- Zheng, Y.; Tian, J.; Kong, X.; Wu, D.; Chen, S.; Liu, D.; Ye, X. Proanthocyanidins from Chinese berry leaves modified the physicochemical properties and digestive characteristic of rice starch. Food Chem. 2021, 335, 127666. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.; Lu, M.; Lu, M.Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D. Phenolic profiles, beta-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Xie, H.; Ju, X.; Liu, R. Phytochemical Profiles and Antioxidant Activity of Adlay Varieties. J. Agric. Food Chem. 2013, 61, 5103–5113. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kong, X.; Zheng, Y.; Sun, W.; Chen, S.; Liu, D.; Zhang, H.; Fang, H.; Tian, J.; Ye, X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason. Sonochem. 2019, 59, 104709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chao, C.; Huang, H.; Wang, S.; Wang, S.; Wang, S.; Copeland, L. Revisiting Mechanisms Underlying Digestion of Starches. J. Agric. Food Chem. 2019, 67, 8212–8226. [Google Scholar] [CrossRef] [PubMed]
- Waleed, A.L.A.; Mahdi, A.A.; Al-Maqtari, Q.A.; Sajid, B.M.; Al-Adeeb, A.; Ahmed, A.; Fan, M.C.; Li, Y.; Qian, H.F.; Liu, J. Characterization of molecular, physicochemical, and morphological properties of starch isolated from germinated highland, barley. Food Biosci. 2021, 42, 101052. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complementary Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). J. Agric. Food Chem. 2006, 54, 7277–7286. [Google Scholar] [CrossRef]
- Obiro, W.; Ray, S.; Emmambux, M. V-amylose structural characteristics, methods of preparation, significance, and potential applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef]
- Hernandez, H.; Gutierrez, T.; Bello-Perez, L. Can starch-polyphenol V-type complexes be considered as resistant starch? Food Hydrocoll. 2022, 124, 107226. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.; Majzne, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A—Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Tian, J.H.; Fang, H.T.; Zhang, H.L.; Kong, X.L.; Wu, D.M.; Zheng, J.Q.; Liu, D.H.; Ye, X.Q.; Chen, S.G.; et al. Physicochemical and digestion properties of potato starch were modified by complexing with grape seed proanthocyanidins. Molecules 2020, 25, 1123. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef]
- Flores-Morales, A.; Jimenez-Estrada, M.; Mora-Escobedo, R. Determination of the structural changes by FT-IR, Raman, and CP/MAS C-13 NMR spectroscopy on retrograded starch of maize tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of alpha-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trend Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Govindaraju, I.; Chakraborty, I.; Baruah, V.; Sarmah, B.; Mahato, K.K.; Mazumder, N. Structure and Morphological Properties of Starch Macromolecule Using Biophysical Techniques. Starch-Starke 2021, 73, 2000030. [Google Scholar] [CrossRef]
- Tan, L.; Kong, L. Starch-guest inclusion complexes: Formation, structure, and enzymatic digestion. Crit. Rev. Food Sci. Nutr. 2020, 60, 780–790. [Google Scholar] [CrossRef]
- Tian, J.; Cai, Y.; Qin, W.; Matsushita, Y.; Ye, X.Q.; Ogawa, Y. Parboiling reduced the crystallinity and in vitro digestibility of non-waxy short grain rice. Food Chem. 2018, 257, 23–28. [Google Scholar] [CrossRef]
- Ou, S.J.L.; Yu, J.; Zhou, W.; Liu, M. Effects of anthocyanins on bread microstructure, and their combined impact on starch digestibility. Food Chem. 2022, 374, 131744. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Bello-Pérez, L. Self-assembled and assembled starch V-type complexes for the development of functional foodstuffs: A review. Food Hydrocoll. 2022, 125, 107453. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Ji, X.; Liu, H.; Liu, N.; Yang, J.; Lu, M.; Han, L.; Wang, M. Evaluation studies on effects of quercetin with different concentrations on the physicochemical properties and in vitro digestibility of Tartary buckwheat starch. Int. J. Biol. Macromol. 2020, 163, 1729–1737. [Google Scholar] [CrossRef]
- Wang, C.; McClements, D.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Resistant starch and its nanoparticles: Recent advances in their green synthesis and application as functional food ingredients and bioactive delivery systems. Trends Food Sci. Technol. 2022, 119, 90–100. [Google Scholar] [CrossRef]
- Svihus, B.; Hervik, A. Digestion and metabolic fates of starch, and its relation to major nutrition-related health problems: A review. Starch-Starke 2016, 68, 302–313. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Yang, X.; Wang, X.; Guo, C.; Sun, L.; Guo, Y. Modulation of gelatinized wheat starch digestion and fermentation profiles by young apple polyphenols in vitro. Food Funct. 2021, 12, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, Q.; Hu, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Physicochemical properties, structure and in vitro digestibility on complex of starch with lotus (Nelumbo nucifera Gaertn.) leaf flavonoids. Food Hydrocoll. 2018, 81, 191–199. [Google Scholar] [CrossRef]
- Hu, X.; Xu, X.; Jin, Z.; Tian, Y.; Bai, X.; Xie, Z. Retrogradation properties of rice starch gelatinized by heat and high hydrostatic pressure (HHP). J. Food Eng. 2011, 106, 262–266. [Google Scholar] [CrossRef]
- Oladele, A.; Duodu, K.; Emmambux, N. Pasting, flow, thermal and molecular properties of maize starch modified with crude phenolic extracts from grape pomace and sorghum bran under alkaline conditions. Food Chem. 2019, 297, 124879. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Gao, W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT—Food Sci. Technol. 2011, 44, 787–792. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]




| Index | Antioxidant Activities (μmol/g) | Phenolic Compounds Content (μmol/g) | Phenolic Compounds Composition (μg/g) | ||||
|---|---|---|---|---|---|---|---|
| FRAP | TEAC | Total Phenolic | Total Flavone | Ferulic Acid | p-Coumaric Acid | Catechin | |
| 80% Methanol | 14.62 ± 0.28 a | 107.10 ± 0.29 b | 4.99 ± 0.29 b | 2.33 ± 0.35 b | 0.848 ± 0.036 a | 0.258 ± 0.021 a | 0.942 ± 0.036 a |
| 80% Ethanol | 16.53 ± 0.26 a | 106.73 ± 0.35 b | 4.42 ± 0.27 b | 3.23 ± 0.11 a | |||
| 80% Acetone | 17.43 ± 1.44 a | 116.30 ± 1.16 a | 12.25 ± 1.13 a | 3.38 ± 0.25 a | 1.152 ± 0.010 b | 0.214 ± 0.017 a | 2.441 ± 0.069 b |
| Pure water | 9.76 ± 1.32 b | 57.17 ± 1.75 c | 2.69 ± 0.19 c | 0.99 ± 0.44 c | |||
| Samples | Binding Ability(%) | 1047/1022 (cm−1) | Digestive Properties | ||
|---|---|---|---|---|---|
| RDS (%) | SDS (%) | RS (%) | |||
| Amylose | 1.378 ± 0.004 a | 41.11 ± 0.99 a | 50.18 ± 1.71 a | 6.15 ± 0.81 ab | |
| MHBP-amylose complexes | 43.14 ± 1.81 a | 1.536 ± 0.003 c | 38.61 ± 0.09 ab | 50.94 ± 1.63 a | 7.64 ± 1.25 ab |
| AHBP-amylose complexes | 56.36 ± 1.94 c | 1.540 ± 0.002 c | 36.17 ± 0.88 b | 47.76 ± 3.32 a | 13.27 ± 2.31 c |
| HBS | 1.435 ± 0.005 b | 39.99 ± 2.23 ab | 52.54 ± 3.36 a | 4.66 ± 2.21 a | |
| MHBP-HBS complexes | 44.74 ± 1.41 a | 1.558 ± 0.003 d | 38.16 ± 2.15 ab | 49.18 ± 1.60 a | 9.88 ± 0.93 bc |
| AHBP-HBS complexes | 52.33 ± 0.90 b | 1.558 ± 0.003 d | 38.34 ± 1.61 ab | 52.83 ± 2.75 a | 6.24 ± 1.04 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Qin, M.; Zhang, M.; Zhang, Y.; Wang, Z.; Liang, S. Highland Barley Polyphenol Delayed the In Vitro Digestibility of Starch and Amylose by Modifying Their Structural Properties. Nutrients 2022, 14, 3743. https://doi.org/10.3390/nu14183743
Ren X, Qin M, Zhang M, Zhang Y, Wang Z, Liang S. Highland Barley Polyphenol Delayed the In Vitro Digestibility of Starch and Amylose by Modifying Their Structural Properties. Nutrients. 2022; 14(18):3743. https://doi.org/10.3390/nu14183743
Chicago/Turabian StyleRen, Xin, Mengyuan Qin, Min Zhang, Yi Zhang, Zhenhua Wang, and Shan Liang. 2022. "Highland Barley Polyphenol Delayed the In Vitro Digestibility of Starch and Amylose by Modifying Their Structural Properties" Nutrients 14, no. 18: 3743. https://doi.org/10.3390/nu14183743
APA StyleRen, X., Qin, M., Zhang, M., Zhang, Y., Wang, Z., & Liang, S. (2022). Highland Barley Polyphenol Delayed the In Vitro Digestibility of Starch and Amylose by Modifying Their Structural Properties. Nutrients, 14(18), 3743. https://doi.org/10.3390/nu14183743