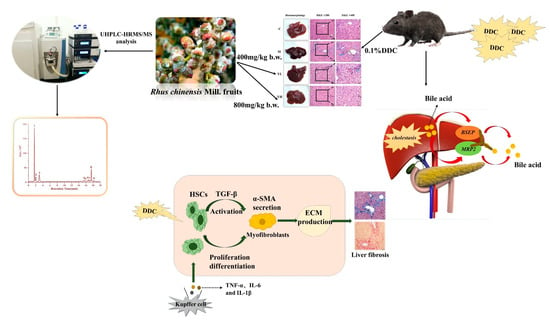
Protective Effect of Rhus chinensis Mill. Fruits on 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine-Induced Cholestasis in Mice via Ameliorating Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation and Phytochemical Characterization of the Sample
2.3. Animal Experimental Design
2.4. Biochemical Analyses
2.5. ELISA Measurements
2.6. Histopathological Assessments
2.7. Western Blot Analyses
2.8. Immunohistochemical (IHC) Analyses
2.9. Statistical Analysis
3. Results
3.1. RCM Alleviated DDC-Induced Liver Injury
3.2. RCM Reduced Oxidative Stress in DDC-Induced Cholestasis
3.3. RCM Inhibited Liver Inflammation in DDC-Induced Cholestasis
3.4. RCM Ameliorated Liver Fibrosis in DDC-Induced Cholestasis
3.5. RCM Improved Bile Duct Proliferation in DDC-Induced Cholestasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UDCA | ursodeoxycholic acid |
| DDC | 3,5-diethoxycarbonyl 1,4-dihydrocoridine |
| CAT | catalase |
| MDA | malondialdehyde |
| GSH | reduced glutathione |
| SOD | superoxide dismutase |
| H&E | hematoxylin-eosin |
| ROS | reactive oxygen species |
| IHC | immunohistochemical |
| SE | standard error |
| TNF-α | tumor necrosis factor-α |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| p-IκBα | phosphonated-inhibitor of nuclear factor kappaB |
| p-NF-κB | phosphorylated-nuclear factor κB |
| TGF-β | transforming growth factor-beta |
| α-SMA | α-Smooth muscle actin |
| BSEP | bile salt export pump |
| MRP2 | multidrug resistance-associated protein 2 |
References
- Schadt, H.S.; Wolf, A.; Pognan, F.; Chibout, S.-D.; Merz, M.; Kullak-Ublick, G.A. Bile acids in drug induced liver injury: Key players and surrogate markers. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolbright, B.L.; Jaeschke, H. Novel insight into mechanisms of cholestatic liver injury. World J. Gastroenterol. 2012, 18, 4985–4993. [Google Scholar] [CrossRef] [PubMed]
- Kriegermeier, A.; Green, R. Pediatric Cholestatic liver disease: Review of bile acid metabolism and discussion of current and emerging therapies. Front. Med. 2020, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Zhang, S.; Lu, Q.; Sun, Y.; Tan, S.; Chen, F.; Tang, L. Protective effects of n-Butanol extract and iridoid glycosides of Veronica ciliata Fisch. Against ANIT-induced cholestatic liver injury in mice. J. Ethnopharmacol. 2022, 266, 113432. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Heathcote, E.J.; Gershwin, M.E. Reviews in basic and clinical gastroenterology and hepatology. Gastroenterology 2010, 139, 1481–1496. [Google Scholar] [CrossRef]
- Šisl, D.; Flegar, D.; Filipović, M.; Turčić, P.; Planinić, P.; Šućur, A.; Kovačić, N.; Grčević, D.; Kelava, T. Tamoxifen ameliorates cholestatic liver fibrosis in mice: Upregulation of TGFβ and IL6 is a potential protective mechanism. Biomedicines 2022, 10, 1209. [Google Scholar] [CrossRef]
- Pose, E.; Sancho-Bru, P.; Coll, M. 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine diet: A rodent model in cholestasis research. In Experimental Cholestasis Research; Humana: New York, NY, USA, 2019; Volume 16, pp. 249–257. [Google Scholar]
- Ali, A.H.; Tabibian, J.H.; Lindor, K.D. Update on pharmacotherapies for cholestatic liver disease. Hepatol. Commun. 2017, 1, 7–17. [Google Scholar] [CrossRef]
- Ommati, M.M.; Farshad, O.; Niknahad, H.; Arabnezhad, M.R.; Azarpira, N.; Mohammadi, H.R.; Haghnegahdar, M.; Mousavi, K.; Akrami, S.; Jamshidzadeh, A.; et al. Cholestasis-associated reproductive toxicity in male and female rats: The fundamental role of mitochondrial impairment and oxidative stress. Toxicol. Lett. 2019, 316, 60–72. [Google Scholar] [CrossRef]
- Kim, T.-W.; Lee, H.-K.; Song, I.-B.; Kim, M.-S.; Hwang, Y.-H.; Lim, J.-H.; Park, S.-J.; Lee, S.-W.; Kim, J.-W.; Yun, H.-I. Protective effect of the aqueous extract from the root of Platycodon grandifforum on cholestasis-induced hepatic injury in mice. Pharm. Biol. 2012, 50, 1473–1478. [Google Scholar] [CrossRef]
- Yu, Y.; Bai, F.; Wang, W.; Liu, Y.; Yuan, Q.; Qu, S.; Zhang, T.; Tian, G.; Li, S.; Li, D.; et al. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol. Biochem. Behav. 2015, 133, 122–131. [Google Scholar] [CrossRef]
- Ye, J.; Piao, H.; Jiang, J.; Jin, G.; Zheng, M.; Yang, J.; Jin, X.; Sun, T.; Choi, Y.H.; Li, L.; et al. Polydatin inhibits mast cell mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci. Rep. 2017, 7, 11895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Liu, Q.; Liu, Q.; Cao, Z.; Zhang, J.; Kuang, T.; Fang, Y.; Liu, G.; Qian, K.; Fu, J.; et al. Camellia oil (Camellia oleifera Abel.) attenuates CCl4-induced liver fibrosis via suppressing hepatocyte apoptosis in mice. Food Funct. 2020, 11, 4582–4590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lin, S.-Y.; Chen, W.-Y.; Liao, S.-L.; Wu, C.-C.; Pan, P.-H.; Chou, S.-T.; Chen, C.-J. Glechoma hederacea extracts attenuate cholestatic liver injury in a bile duct-ligated rat model. J. Ethnopharmacol. 2017, 204, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Cao, P.; Yin, Z.; Wang, X.; Chen, Y.; Yu, M.; Xu, B.; Liao, C.; Duan, Y.; Zhang, S.; et al. Apigenin protects mice against 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced cholestasis. Food Funct. 2021, 12, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Soroka, C.J.; Boyer, J.L. The bile salt export pump: Clinical and experimental aspects of genetic and acquired cholestatic liver disease. Semin. Liver Dis. 2010, 30, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.-Y.; Ai, G.; Zhang, X.-J.; Xu, H.-J.; Huang, Z.-M. Investigations of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) medic against α-naphthylisothiocyanate-induced cholestatic liver injury in rats. J. Ethnopharmacol. 2015, 172, 202–213. [Google Scholar] [CrossRef]
- Ma, N.; Sun, Y.; Yi, J.; Zhou, L.; Cai, S. Chinese sumac (Rhus chinensis Mill.) fruits alleviate indomethacin-induced gastric ulcer in mice by improving oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2022, 284, 114752. [Google Scholar] [CrossRef]
- Li, M.; Wang, A.; Zhang, Y.; Han, T.; Guan, L.; Fan, D.; Liu, J.; Xu, Y. A comprehensive review on ethnobotanical, phytochemical and pharmacological aspects of Rhus chinensis Mill. J. Ethnopharmacol. 2017, 204, 58–66. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, N.; Yi, J.; Zhou, L.; Cai, S. Gastroprotective effect and mechanisms of Chinese sumac fruits (Rhus chinensis Mill.) on ethanol-induced gastric ulcers in mice. Food Funct. 2021, 12, 12565–12579. [Google Scholar] [CrossRef]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis–folklore to modern evidence. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhao, Y.; Hong, Y.; Cai, S.; Pang, M. Phenolic composition, antioxidant and pancreatic lipase inhibitory activities of Chinese sumac (Rhus chinensis Mill.) fruits extracted by different solvents and interaction between myricetin-3-O-rhamnoside and quercetin-3-O-rhamnoside. Int. J. Food Sci. Technol. 2018, 53, 1045–1053. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, N.; Liu, X.; Yi, Y.; Cai, S. Preventive effects of Chinese sumac fruits against acetaminophen-induced liver injury in mice via regulating oxidative stress, inflammation and apoptosis. J. Funct. Foods 2021, 87, 104830. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Gong, X.; Zhang, Y.; Zhao, L.; Cheng, G.; Cai, S. Rhus chinensis Mill. fruits prevent high-fat/ethanol diet-induced alcoholic fatty liver in rats via AMPK/SREBP-1/FAS signaling pathway. J. Funct. Foods 2019, 61, 103498. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Chen, T.; Cheng, G.; Cai, S. Preventive effect of ethanol extract from Chinese sumac fruits against tetrachloromethane-induced liver fibrosis in mice. Food Funct. 2020, 11, 7061–7072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, L.; Wang, Y.; Zhao, T.; Khan, A.; Wang, Y.; Cao, J.; Cheng, J. Protective effect of Que Zui tea hot-water and aqueous ethanol extract against acetaminophen-induced liver injury in mice via inhibition of oxidative stress, inflammation, and apoptosis. Food Funct. 2021, 12, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- González-Ponce, H.A.; González-Ponce, M.C.; Tepper, P.G.; Quax, W.J.; Buist-Homan, M.; NicoFaber, K.; Moshage, H. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res. Int. 2020, 137, 109461. [Google Scholar] [CrossRef]
- Qiu, Z.; Tang, M.; Deng, G.; Yang, H.; Zhang, X.; Huang, S.; Wu, L. Antioxidant and antigenotoxic activities of ethanol extracts from Rhus chinensis Mill Leaves. Food Sci. Biotechnol. 2014, 23, 1213–1221. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Ouyang, X.; Chen, Y.; Soroka, C.-J.; Wang, J.; Mennone, A.; Wang, Y.; Mehal, W.-Z.; Jain, D.; Boyer, J.-L. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017, 2, e90780. [Google Scholar] [CrossRef] [Green Version]
- Copple, B.-L.; Jaeschke, H.; Klaassen, C.-D. Oxidative stress and the pathogenesis of cholestasis. Semin. Liver Dis. 2010, 30, 195–204. [Google Scholar] [CrossRef]
- Salunga, T.-L.; Cui, Z.-G.; Shimoda, S.; Zheng, H.-C.; Nomoto, K.; Kondo, T.; Takano, Y.; Selmi, C.; Alpini, G.; Gershwin, M.E.; et al. Oxidative stress-induced apoptosis of bile duct cells in primary biliary cirrhosis. J. Autoimmun. 2007, 29, 78–86. [Google Scholar] [CrossRef]
- Mariotti, V.; Strazzabosco, M.; Fabris, L.; Calvisi, D.F. Animal models of biliary injury and altered bile acid metabolism. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fan, X.; Feng, Z.; Ma, Y.; Lan, X.; Chen, M. Ethyl acetate extract of herpetospermum pedunculosum alleviates α-naphthylisothiocyanate-induced cholestasis by activating the farnesoid x-receptor and suppressing oxidative stress and inflammation in rats. Phytomedicine 2020, 76, 153257. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Mann, D.A. Role of nuclear factor κB in liver health and disease. Clin. Sci. 2010, 118, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yang, T.-H.; Cho, H.-Y. Antiffbrotic effects of green tea on in vitro and in vivo models of liver fibrosis. World J. Gastroenterol. 2009, 15, 5200. [Google Scholar] [CrossRef]
- Kim, J.-Y.; An, H.-J.; Kim, W.-H.; Park, Y.-Y.; Park, K.-D.; Park, K.-K. Apamin suppresses biliary ffbrosis and activation of hepatic stellate cells. Int. J. Mol. Med. 2017, 39, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Garcés, V.; Harvat, M.; Molina Aguilar, P.; Ferrandez Izquierdo, A.; Ruiz-Saurí, A. Comparative measurement of collagen bundle orientation by Fourier analysis and semiquantitative evaluation: Reliability and agreement in Masson’s trichrome, Picrosirius red and confocal microscopy techniques. J. Microsc. 2017, 267, 130–142. [Google Scholar] [CrossRef]
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP imbalance and collagen production induced by IL-1β or TNF-α release from human hepatic stellate cells. PLoS ONE 2016, 11, e0153118. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-L.; Lin, Y.-T.; Kung, H.-N.; Hou, Y.-C.; Liu, J.-J.; Pan, M.-H.; Chen, H.-L.; Yu, C.-H.; Tsai, P.-J. A triterpenoid-enriched extract of bitter melon leaves alleviates hepatic fibrosis by inhibiting inflammatory responses in carbon tetrachloride-treated mice. Food Funct. 2021, 12, 7805–7815. [Google Scholar] [CrossRef]
- Bourebaba, N.; Marycz, K. Hepatic stellate cells role in the course of metabolic disorders development—A molecular overview. Pharmacol. Res. 2021, 170, 105739. [Google Scholar] [CrossRef]
- Hu, B.; Mao, Z.; Jiang, X.; He, D.; Wang, Z.; Wang, X.; Zhu, Y.; Wang, H. Role of TGF-β1/Smad3-mediated fibrosis in drug resistance mechanism of prolactinoma. Brain Res. 2018, 1698, 204–212. [Google Scholar] [CrossRef]
- Li, W.K.; Wang, G.F.; Wang, T.M.; Li, Y.Y.; Li, Y.F.; Lu, X.Y.; Wang, Y.H.; Zhang, H.; Liu, P.; Wu, J.S.; et al. Protective effect of herbal medicine Huangqi decoction against chronic cholestatic liver injury by inhibiting bile acid-stimulated inflammation in DDC-induced mice. Phytomedicine 2019, 62, 152948. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, L.; Shan, W.; Kong, L.; Chen, N.; Lou, Y.; Zeng, S. The role of the sodium-taurocholate co-transporting polypeptide (NTCP) and bile salt export pump (BSEP) in related liver disease. Curr. Drug Metab. 2019, 20, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Deferm, N.; Vocht, T.D.; Qi, B.; Brantegem, P.V.; Gijbels, E.; Vinken, M.; Witte, P.; Bouillon, T.; Annaert, P. Current insights in the complexities underlying drug-induced cholestasis. Crit. Rev. Toxicol. 2019, 49, 520–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.-J.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xiang, J.; Huang, G.; Zhao, Y.; Wang, X.; Wu, H.; Jiang, K.; Liang, Z.; Kang, L.; Yang, G.; et al. Pterostilbene alleviates cholestasis by promoting SIRT1 activity in hepatocytes and macrophages. Front. Pharmacol. 2021, 12, 785403. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Wu, B.; Wang, Y.; Song, F.; Gu, Y.; Yuan, Y. Salvianolic acid B protects against ANIT-induced cholestatic liver injury through regulating bile acid transporters and enzymes, and NF-κB/IκB and MAPK pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1169–1180 . [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Cai, S. The preventive effect and underlying mechanism of Rhus chinensis Mill. fruits on dextran sulphate sodium-induced ulcerative colitis in mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversation between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Cai, S.; Zhang, Y.; Ma, N.; Yi, J.; Hu, X.; Wang, T. Protective Effect of Rhus chinensis Mill. Fruits on 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine-Induced Cholestasis in Mice via Ameliorating Oxidative Stress and Inflammation. Nutrients 2022, 14, 4090. https://doi.org/10.3390/nu14194090
Sun Y, Cai S, Zhang Y, Ma N, Yi J, Hu X, Wang T. Protective Effect of Rhus chinensis Mill. Fruits on 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine-Induced Cholestasis in Mice via Ameliorating Oxidative Stress and Inflammation. Nutrients. 2022; 14(19):4090. https://doi.org/10.3390/nu14194090
Chicago/Turabian StyleSun, Yilin, Shengbao Cai, Yuanyue Zhang, Nan Ma, Junjie Yi, Xiaosong Hu, and Tao Wang. 2022. "Protective Effect of Rhus chinensis Mill. Fruits on 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine-Induced Cholestasis in Mice via Ameliorating Oxidative Stress and Inflammation" Nutrients 14, no. 19: 4090. https://doi.org/10.3390/nu14194090
APA StyleSun, Y., Cai, S., Zhang, Y., Ma, N., Yi, J., Hu, X., & Wang, T. (2022). Protective Effect of Rhus chinensis Mill. Fruits on 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine-Induced Cholestasis in Mice via Ameliorating Oxidative Stress and Inflammation. Nutrients, 14(19), 4090. https://doi.org/10.3390/nu14194090