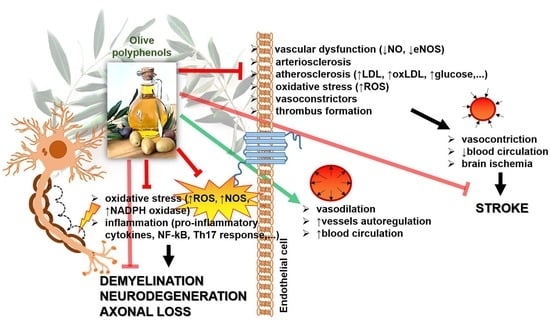
Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties
Abstract
:1. Introduction
2. Methods
3. Anti-Demyelination Action of Olive Polyphenols
3.1. Olive Polyphenols and Pro-Inflammatory Cytokine Release
3.2. Olive Polyphenols and Immune Cells
3.3. Olive Polyphenols and Oxidative Stress
4. Anti-Stroke Action of Olive Polyphenols
4.1. Olive Polyphenols and Hypertension
4.2. Olive Polyphenols and Vascular Dysfunction
4.2.1. Olive Polyphenols and Atherosclerosis
4.2.2. Olive Polyphenols and Hyperlipidemia
4.2.3. Olive Polyphenols and Hyperglycemia
4.2.4. Olive Polyphenols and Thrombosis (Platelet Aggregation)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of Bioactive Compounds in Commercial Olive Leaf Extracts, and Olive Leaves and their Infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/herbal/oleae-folium (accessed on 15 October 2022).
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary Polyphenols: Structures, Bioavailability and Protective Effects Against Atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Antioxidant Oleuropein: Exceeding the Expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [Green Version]
- Casamenti, F.; Stefani, M. Olive Polyphenols: New Promising Agents to Combat Aging-Associated Neurodegeneration. Expert Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef]
- Fabiani, R. Anti-Cancer Properties of Olive Oil Secoiridoid Phenols: A Systematic Review of in Vivo Studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; de Wiele, T.V.; Possemiers, S. Gut Metabotypes Govern Health Effects of Dietary Polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of Gut Microbiota with Functional Food Components and Nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive Oil Phenols are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, C.; Galletti, P.; Maisto, G.; Cucciolla, V.; D’Angelo, S.; Zappia, V. Transport Mechanism and Metabolsi of Olive Oil Hydroxytyrosol in Caco-2 Cells. FEBS Lett. 2000, 470, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in Vivo Fate of Hydroxytyrosol and Tyrosol, Antioxidant Phenolic Constituents of Olive Oil, after Intravenous and Oral Dosing of Labeled Compounds to Rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Tzounis, X.; Dessì, M.A.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The Fate of Olive Oil Polyphenols in the Gastrointestinal Tract: Implications of Gastric and Colonic Microflora-Dependent Biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Physiological Linkage of Gender, Bioavailable Hydroxytyrosol Derivatives, and their Metabolites with Systemic Catecholamine Metabolism. Food Funct. 2017, 8, 4570–4581. [Google Scholar] [CrossRef]
- de las Hazas, M.-C.L.; Piñol, C.; Macià, A.; Romero, M.-P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.-J. Differential Absorption and Metabolism of Hydroxytyrosol and its Precursors Oleuropein and Secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Magyari, M.M. Apparent Changes in the Epidemiology and Severity of Multiple Sclerosis. Nat. Rev. Neurol. 2021, 17, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.S. Progress-Defining Risk Factors for Stroke Prevention. Cerebrovasc. Dis. 2021, 50, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef]
- Ivanov, M.; Vajic, U.-J.; Mihailovic-Stanojevic, N.; Miloradovic, Z.; Jovovic, D.; Grujic-Milanovic, J.; Karanovic, D.; Dekanski, D. Highly Potent Antioxidant Olea europaea L. Leaf Extract Affects Carotid and Renal Haemodynamics in Experimental Hypertension: The Role of Oleuropein. Excli J. 2018, 17, 29–44. [Google Scholar] [CrossRef]
- Menezes, R.C.R.; Peres, K.K.; Costa-Valle, M.T.; Faccioli, L.S.; Dallegrave, E.; Garavaglia, J.; Bosco, S.M.D. Oral Administration of Oleuropein and Olive Leaf Extract has Cardioprotective Effects in Rodents: A Systematic Review. Rev. Port. Cardiol. 2022, 41, 167–175. [Google Scholar] [CrossRef]
- Patti, A.M.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Nikolic, D.; Giglio, R.V.; Terranova, A.; Soresi, M.; Giannitrapani, L.; Montalto, G.; et al. Daily Use of Extra Virgin Olive Oil with High Oleocanthal Concentration Reduced Body Weight, Waist Circumference, Alanine Transaminase, Inflammatory Cytokines and Hepatic Steatosis in Subjects with the Metabolic Syndrome: A 2-Month Intervention Study. Metabolites 2020, 10, 392. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Vazquez-Aguilar, L.A.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Espejo-Calvo, J.A.; Olmo-Peinado, J.M.; de la Torre, R.; Fito-Colomer, M.; Covas, M.I.; Romero, C.; et al. May Bioactive Compounds from the Olive Fruit Improve the Postprandial Insulin Response in Healthy Adults? J. Funct. Foods 2021, 83, 104561. [Google Scholar] [CrossRef]
- Varas, R.; Ortiz, F.C. Neuroinflammation in Demyelinating Diseases: Oxidative Stress as a Modulator of Glial Crosstalk. Curr. Pharm. Des. 2019, 25, 4755–4762. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple Sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function-A systematic review and meta-analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef] [Green Version]
- Gorzynik-Debicka, M.; Przychodzen, P.; Capello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 547. [Google Scholar] [CrossRef] [Green Version]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavales, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols Journey through Blood-brain Barrier towards Neuronal Protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, J.; Kezele, T.G. Olive Leaf Polyphenols Attenuate the Clinical Course of Experimental Autoimmune Encephalomyelitis and Provide Neuroprotection by Reducing Oxidative Stress, Regulating Microglia and SIRT1, and Preserving Myelin Integrity. Oxid. Med. Cell. Longev. 2020, 2020, 6125638. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-KappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Fazal, F. Blocking NF-κB: An Inflammatory Issue. Proc. Am. Thorac. Soc. 2011, 8, 497–503. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H.; Marchesi, V.V.; Šain, I.; Romić, Ž.; Rahelić, D. Preventive and Therapeutic Effects of Oleuropein Against Carbon Tetrachloride-Induced Liver Damage in Mice. Pharmacol. Res. 2012, 65, 451–464. [Google Scholar] [CrossRef]
- Yue, Y.; Stone, S.; Lin, W. Role of Nuclear Factor κB in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Neural Regen. Res. 2018, 13, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Min, J.S.; Chae, U.; Lee, J.Y.; Song, K.-S.; Lee, H.-S.; Lee, H.J.; Lee, S.-R.; Lee, D.-S. Anti-Inflammatory Effect of Oleuropein on Microglia through Regulation of Drp1-Dependent Mitochondrial Fission. J. Neuroimmunol. 2017, 306, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Che, S.; Zhu, M. Oleuropein Improves Cognitive Dysfunction and Neuroinflammation in Diabetic Rats through the PI3K/Akt/mTOR Pathway. Appl. Bionics Biomech. 2022, 2022, 5892463. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.; Podojil, J.R.; McCarthy, D.P.; Miller, S.D.; Popko, B. Oligodendrocyte Death Results in Immune-Mediated CNS Demyelination. Nat. Neurosci. 2016, 19, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.D.; Miller, J.F.A.P. Discovery of 2 Distinctive Lineages of Lymphocytes, T Cells and B Cells, as the Basis of the Adaptive Immune System and Immunologic Function: 2019 Albert Lasker Basic Medical Research Award. JAMA 2019, 322, 1247–1248. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Dhaheri, A.S.A.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Ballke, C.; Gran, E.; Baekkevold, E.S.; Jahnsen, F.L. Characterization of Regulatory T-cell Markers in CD4+ T Cells of the Upper Airway Mucosa. PLoS ONE 2016, 11, e0148826. [Google Scholar] [CrossRef]
- Gorenec, L.; Lepej, S.Z.; Grgic, I.; Planinic, A.; Bes, J.I.; Vince, A.; Begovac, J. The Comparison of Th1, Th2, Th9, Th17 and Th22 Cytokine Profiles in Acute and Chronic HIV-1 Infection. Microb. Pathog. 2016, 97, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Semin. Neurol. 2016, 36, 115–127. [Google Scholar] [CrossRef]
- Yshii, L.; Gebauer, C.; Bernard-Valnet, R.; Liblau, R. Neurons and T cells: Understanding this Interaction for Inflammatory Neurological Diseases. Eur. J. Immunol. 2015, 45, 2712–2720. [Google Scholar] [CrossRef]
- Miljkovic, D.J.; Dekanski, D.; Miljkovic, Z.; Momcilovic, M.; Mostarica-Stojkovic, M. Dry Olive Leaf Extract Ameliorates Experimental Autoimmune Encephalomyelitis. Clin. Nutr. 2009, 28, 3462013350. [Google Scholar] [CrossRef] [PubMed]
- Cvjetićanin, T.; Miljković, D.; Stojanović, I.; Dekanski, D.; Stošić-Grujičić, S. Dried Leaf Extract of Olea Europaea Ameliorates Islet-Directed Autoimmunity in Mice. Br. J. Nutr. 2010, 103, 1413–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amici, S.A.; Dong, J.; Guerau-de-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano, R.; Millan-Linares, M.C.; Naranjo, M.C.; Lemus-Conejo, A.; Claro, C.; la Paz, S.M.D. Unsaponifiable and Phenolic Fractions From Virgin Olive Oil Prevent Neuroinflammation Skewing Microglia Polarization Toward M2 Phenotype. J. Funct. Foods 2019, 62, 103543. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, Y.Z.; Wang, G.Q.; Li, D.D.; Shi, J.S.; Zhang, F. Targeting MAPK Pathways by Naringenin Modulates Microglia M1/M2 Polarization in Lipopolysaccharide-Stimulated Cultures. Front. Cell Neurosci. 2018, 12, 531. [Google Scholar] [CrossRef] [Green Version]
- Chitnis, T.; Weiner, H.L. CNS Inflammation and Neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [Green Version]
- Taticchi, A.; Urbani, S.; Albi, E.; Servilli, M.; Codini, M.; Traina, G.; Balloni, S.; Patria, F.F.; Perioli, L.; Beccari, T.; et al. In Vitro Anti-Inflammatory Effects of Phenolic Compounds from Moraiolo Virgin Olive Oil (MVOO) in Brain Cells via Regulating the TLR4/NLRP3 Axis. Molecules 2019, 24, 4523. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Ljubisavljevic, S. Oxidative Stress and Neurobiology of Demyelination. Mol. Neurobiol. 2016, 53, 744–758. [Google Scholar] [CrossRef]
- Michaličková, D.; Šíma, M.; Slanař, O. New Insights in the Mechanisms of Impaired Redox Signaling and its Interplay with Inflammation and Immunity in Multiple Sclerosis. Physiol. Res. 2020, 69, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross Talk in Alzheimer’s Disease and Parkinson’s Disease. Drug Des. Dev. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-Like Activity in Extra-Virgin Olive Oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotech. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Tariq, U.; Haq, I.-U.; Naz, A.; Rizwan, M. Neuroprotective Effects of Oleuropein: Recent Developments and Contemporary Research. J. Food Biochem. 2021, 45, e13967. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, M.; Kiss, A.K.; Naruszewicz, M. A Comparison of Antioxidant Activities of Oleuropein and its Dialdehydic Derivative from Olive Oil, Oleacin. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and Antioxidant Effects on Breast Cancer Cells of Oleuropein and its Semisynthetic Peracetylated Derivatives. Food Chem. 2011, 127, 1609–1614. [Google Scholar] [CrossRef]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millán, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A.; de Castro, M.D.L. A Pilot Study on the DNA-Protective, Cytotoxic, and Apoptosis-Inducing Properties of Olive-Leaf Extracts. Mutat. Res. 2011, 723, 165–170. [Google Scholar] [CrossRef]
- Benedetto, R.D.; Van, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, The Major Extra Virgin Olive Oil Compound, Restored Intracellular Antioxidant Defences In Spite Of Its Weak Antioxidative Effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef]
- Hernandes, M.S.; Britto, L.R. NADPH Oxidase and Neurodegeneration. Curr. Neuropharmacol. 2012, 10, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ćurko-Cofek, B.; Kezele, T.G.; Marinić, J.; Tota, M.; Čizmarević, N.S.; Milin, Č.; Ristić, S.; Radošević-Stašić, B.; Barac-Latas, V. Chronic Iron Overload Induces Gender-Dependent Changes in Iron Homeostasis, Lipid Peroxidation and Clinical Course of Experimental Autoimmune Encephalomyelitis. Neurotoxicology 2016, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ćurko-Cofek, B.; Kezele, T.G.; Barac-Latas, V. Hepcidin and Metallothioneins as Molecular Base for Sex-Dependent Differences in Clinical Course of Experimental Autoimmune Encephalomyelitis in Chronic Iron Overload. Med. Hypotheses 2017, 107, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Nunez, M.T.; Urrutia, P.; Mena, N.; Aguirre, P.; Tapia, V.; Salazar, J. Iron Toxicity in Neurodegeneration. Biometals 2012, 25, 761–776. [Google Scholar] [CrossRef]
- Hametner, S.; Wimmer, I.; Haider, L.; Pfeifenbring, S.; Bruck, W.; Lassmann, H. Iron and Neurodegeneration in the Multiple Sclerosis Brain. Ann. Neurol. 2013, 74, 848–861. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-Rich Olive Mill Wastewater Extract Protects Brain Cells In Vitro and Ex Vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef]
- Samieri, C.; Feart, C.; Proust-Lima, C.; Peuchant, E.; Tzourio, C.; Stapf, C.; Berr, C.; Barberger-Gateau, P. Olive Oil Consumption, Plasma Oleic Acid, and Stroke Incidence: The Three-City Study. Neurology 2011, 77, 418–425. [Google Scholar] [CrossRef]
- Giacometti, J.; Muhvić, D.; Grubić-Kezele, T.; Nikolić, M.; Šoić-Vranić, T.; Bajek, S. Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats. Int. J. Mol. Sci. 2020, 21, 8981. [Google Scholar] [CrossRef]
- Johansson, B.B. Vascular Mechanisms in Hypertensive Cerebrovascular Disease. J. Cardiovasc. Pharmacol. 1992, 19, 11–15. [Google Scholar] [CrossRef]
- Oh, Y.S. Arterial Stiffness and Hypertension. Clin. Hypertens. 2018, 24, 17. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B.; Fredriksson, K. Cerebral Arteries in Hypertension: Structural and Hemodynamic Aspects. J. Cardiovasc. Pharmacol. 1985, 7, 90–93. [Google Scholar] [CrossRef]
- Johansson, B.B. Hypertension Mechanisms Causing Stroke. Clin. Exp. Pharmacol. Physiol. 1999, 26, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial Function in Cardiovascular Medicine: A Consensus Paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Barbero, N.; Gutiérrez-Muñoz, C.; Blanco-Colio, L.M. Cellular Crosstalk between Endothelial and Smooth Muscle Cells in Vascular Wall Remodeling. Int. J. Mol. Sci. 2021, 22, 7284. [Google Scholar] [CrossRef]
- Murray, E.C.; Nosalski, R.; MacRitchie, N.; Tomaszewski, M.; Maffia, P.; Harrison, D.G.; Guzik, T.J. Therapeutic Targeting of Inflammation in Hypertension: From Novel Mechanisms to Translational Perspective. Cardiovasc. Res. 2021, 117, 2589–2609. [Google Scholar] [CrossRef]
- Fernández-Castillejo, S.; García-Heredia, A.I.; Solà, R.; Camps, J.; de la Hazas, M.C.L.; Farràs, M.; Pedret, A.; Catalán, Ú.; Rubió, L.; Motilva, M.J.; et al. Phenol-Enriched Olive Oils Modify Paraoxonase-Related Variables: A Randomized, Crossover, Controlled Trial. Mol. Nutr. Food. Res. 2017, 61, 1600932. [Google Scholar] [CrossRef]
- Vazquez, A.; Sanchez-Rodriguez, E.; Vargas, F.; Montoro-Molina, S.; Romero, M.; Espejo-Calvo, J.A.; Vilchez, P.; Jaramillo, S.; Olmo-García, L.; Carrasco-Pancorbo, A.; et al. Cardioprotective Effect of a Virgin Olive Oil Enriched with Bioactive Compounds in Spontaneously Hypertensive Rats. Nutrients 2019, 11, 1728. [Google Scholar] [CrossRef] [Green Version]
- Rahimianfar, F. The Effect of Olive Leaf Extract on Systolic and Diastolic Blood Pressure in Adults: A Systemic Review and Meta-Analysis. In Olive Cultivation; Yonar, T., Ed.; Intechopen: London, UK, 2022. [Google Scholar] [CrossRef]
- Perrinjaquet-Moccetti, T.; Busjahn, A.; Schmidlin, C.; Schmidt, A.; Bradl, B.; Aydogan, C. Food Supplementation with an Olive (Olea europaea L.) Leaf Extract Reduces Blood Pressure in Borderline Hypertensive Monozygotic Twins. Phytother. Res. 2008, 22, 1239–1242. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.G.; Villar, J.; Stiefel, P. Olive Oil Polyphenols Decrease Blood Pressure and Improve Endothelial Function in Young Women with Mild Hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozati, M.; Barnett, J.; Wu, D.; Handelman, G.; Saltzman, E.; Wilson, T.; Li, L.; Wang, J.; Marcos, A.; Ordovás, J.M.; et al. Cardio-Metabolic and Immunological Impacts of Extra Virgin Olive Oil Consumption in Overweight and Obese Older Adults: A Randomized Controlled Trial. Nutr. Metab. 2015, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of Phenolic-Rich Olive Leaf EExtract on Blood Pressure, Plasma Lipids and Inflammatory Markers: A Randomised Controlled Trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermans, M.P.; Lempereur, P.; Salembier, J.-P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts in the Clinical Management of Hypertension and Metabolic Syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Ismail, M.A.; Norhayati, M.N.; Mohamad, N.N. Olive Leaf Extract Effect on Cardiometabolic Profile Among Adults with Prehypertension and Hypertension: A Systematic Review and Meta-Analysis. Peer J. 2021, 9, e11173. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Roselló-Catafau, J.; Pintó, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol Fraction of Extra Virgin Olive Oil Protects Against Endothelial Dysfunction Induced by High Glucose and Free Fatty Acids Through Modulation of Nitric Oxide and Endothelin-1. Redox Biol. 2014, 2C, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Yun, J.; Kwon, S.-M. Therapeutic Strategies for Oxidative Stress-Related Cardiovascular Diseases: Removal of Excess Reactive Oxygen Species in Adult Stem Cells. Oxid. Med. Cell Longev. 2016, 2016, 2483163. [Google Scholar] [CrossRef] [Green Version]
- Nasrallah, H.; Aissa, I.; Slim, C.; Boujbiha, M.A.; Zaouali, M.A.; Bejaoui, M.; Wilke, V.; Jannet, H.B.; Mosbah, H.; Abdennebi, H.B. Effect of Oleuropein on Oxidative Stress, Inflammation and Apoptosis Induced by Ischemia-Reperfusion Injury in Rat Kidney. Life Sci. 2020, 255, 117833. [Google Scholar] [CrossRef]
- Motawea, M.H.; Elmaksoud, H.A.A.; Elharrif, M.G.; Desoky, A.A.E.; Ibrahimi, A. Evaluation of Anti-inflammatory and Antioxidant Profile of Oleuropein in Experimentally Induced Ulcerative Colitis. Int. J. Mol. Cell Med. 2020, 9, 224–233. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Yazid, M.D.; Idrus, R.B.H.; Rahman, M.R.A.; Sulaiman, N. Molecular Action of Hydroxytyrosol in Attenuation of Intimal Hyperplasia: A Scoping Review. Front. Pharmacol. 2021, 12, 663266. [Google Scholar] [CrossRef]
- Mizutani, D.; Onuma, T.; Tanabe, K.; Kojima, A.; Uematsu, K.; Nakashima, D.; Doi, T.; Enomoto, Y.; Matsushima-Nishiwaki, R.; Tokuda, H.; et al. Olive Polyphenol Reduces the Collagen-Elicited Release of Phosphorylated HSP27 from Human Platelets. Biosci. Biotechnol. Biochem. 2020, 84, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Burja, B.; Kuret, T.; Janko, T.; Topalović, D.; Živković, L.; Mrak-Poljšak, K.; Spremo-Potparević, B.; Žigon, P.; Distler, O.; Saša Čučnik, S.; et al. Olive Leaf Extract Attenuates Inflammatory Activation and DNA Damage in Human Arterial Endothelial Cells. Front. Cardiovasc. Med. 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Hedström, D.; García-Villalón, A.L.; Amor, S.; de la Fuente-Fernández, M.; Almodóvar, P.; Prodanov, M.; Priego, T.; Martín, A.I.; Inarejos-García, A.M.; Granado, M. Olive Leaf Extract Supplementation Improves the Vascular and Metabolic Alterations Associated with Aging in Wistar Rats. Sci. Rep. 2021, 11, 8188. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Hori, K.; Tokutake, Y.; Hakamata, Y.; Kawabata, F.; Toyomizu, M.; Kikusato, M. Oleuropein Suppresses Mitochondrial Reactive Oxygen Species Generation Possibly Via an Activation of Transient Receptor Potential V1 and Sirtuin-1 in Cultured Chicken Muscle Cells. Anim. Sci. J. 2022, 93, e13677. [Google Scholar] [CrossRef] [PubMed]
- Torul, H.; Küçükboyacı, N.; Tamer, U.; Karasu, Ç. Evaluation of Phenolic Compounds and Protective Effects of Olive (Olea europaea L.) Leaf Extracts on Endothelial Cells Against Hydrogen Peroxide-Induced Toxicity. J. Res. Pharm. 2020, 24, 497–507. [Google Scholar] [CrossRef]
- Choi, S.H.; Joo, H.B.; Lee, S.J.; Choi, H.Y.; Park, J.H.; Baek, S.H.; Kwon, S.M. Oleuropein Prevents Angiotensin II-Mediated Human Vascular Progenitor Cell Depletion. Int. J. Cardiol. 2015, 181, 160–165. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Piscopo, A.; Abdel-Rahman, W.M.; Piga, A.; Pintus, G. Antioxidant Properties of Olive Mill Wastewater Polyphenolic Extracts on Human Endothelial and Vascular Smooth Muscle Cells. Foods 2021, 10, 800. [Google Scholar] [CrossRef]
- Nekooeian, A.A.; Khalili, A.; Khosravi, M.B. Effects of Oleuropein in Rats With Simultaneous Type 2 Diabetes and Renal Hypertension: A Study of Antihypertensive Mechanisms. J. Asian Nat. Prod. Res. 2014, 16, 953–962. [Google Scholar] [CrossRef]
- Ilic, S.; Stojiljkovic, N.; Stojanovic, N.; Stoiljkovic, M.; Mitic, K.; Salinger-Martinovic, S.; Randjelovic, P. Effects of Oleuropein on Rat’s Atria and Thoracic Aorta: A Study of Antihypertensive Mechanisms. Can. J. Physiol. Pharmacol. 2021, 99, 110–114. [Google Scholar] [CrossRef]
- Alcaide-Hidalgo, J.M.; Romero, M.; Duarte, J.; López-Huertas, E. Antihypertensive Effects of Virgin Olive Oil (Unfiltered) Low Molecular Weight Peptides with ACE Inhibitory Activity in Spontaneously Hypertensive Rats. Nutrients 2020, 12, 271. [Google Scholar] [CrossRef] [Green Version]
- Alcaide-Hidalgo, J.M.; Margalef, M.; Bravo, F.I.; Muguerza, B.; López-Huertas, E. Virgin Olive Oil (Unfiltered) Extract Contains Peptides and Possesses ACE Inhibitory and Antihypertensive Activity. Clin. Nutr. 2020, 39, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease: From Marvel to Menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; de Sotomayor, M.A.; Ruiz-Gutierrez, V. Pomace Olive Oil Improves Endothelial Function in Spontaneously Hypertensive Rats by Increasing Endothelial Nitric Oxide Synthase Expression. Am. J. Hypertens. 2007, 20, 728–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serreli, G.; Sayec, M.L.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G.G. Conjugated Metabolites of Hydroxytyrosol and Tyrosol Contribute to the Maintenance of Nitric Oxide Balance in Human Aortic Endothelial Cells at Physiologically Relevant Concentrations. Molecules 2021, 26, 7480. [Google Scholar] [CrossRef]
- Ranieri, M.; Mise, A.D.; Centrone, M.; D’Agostino, M.; Tingskov, S.J.; Venneri, M.M.; Pellegrino, T.; Difonzo, G.; Caponio, F.; Norregaard, R.; et al. Olive Leaf Extract (OLE) Impaired Vasopressin-Induced Aquaporin-2 Trafficking through the Activation of the Calcium-Sensing Receptor. Sci. Rep. 2021, 11, 4537. [Google Scholar] [CrossRef] [PubMed]
- Ghibu, S.; Morgovan, C.; Vostinaru, O.; Olah, N.; Mogosan, C.; Muresan, A. 0347: Diuretic, antihypertensive and antioxidant effect of olea europaea leaves extract, in rats. Arch. Cardiovasc. Dis. Suppl. 2015, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Matjuda, E.N.; Engwa, G.A.; Sewani-Rusike, C.R.; Nkeh-Chungag, B.N. An Overview of Vascular Dysfunction and Determinants: The Case of Children of African Ancestry. Front. Pediatr. 2021, 9, 769589. [Google Scholar] [CrossRef]
- van Sloten, T.T. Vascular dysfunction: At the heart of cardiovascular disease, cognitive impairment and depressive symptoms. Artery Res. 2017, 19, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of Arterial Stiffening: From Mechanotransduction to Epigenetics. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1055–1062. [Google Scholar] [CrossRef]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front. Pharmacol. 2021, 12, 787541. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- Summerhill, V.; Karagodin, V.; Grechko, A.; Myasoedova, V.; Orekhov, A. Vasculoprotective Role of Olive Oil Compounds via Modulation of Oxidative Stress in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, F. Polyphenols can Potentially Prevent Atherosclerosis and Cardiovascular Disease by Modulating Macrophage Cholesterol Metabolism. Curr. Mol. Pharmacol. 2021, 14, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Covas, M.-I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.-F.; de la Torre, R.; Muñoz-Aguayo, D.; Vila, J.; Fitó, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244. [Google Scholar] [CrossRef] [Green Version]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Castillejo, S.; Valls, R.-M.; Castañer, O.; Rubió, L.; Catalán, U.; Pedret, A.; Macià, A.; Sampson, M.L.; Covas, M.-I.; Fitó, M. Polyphenol rich olive oils improve lipoprotein particle atherogenic ratios and subclasses profile: A randomized, crossover, controlled trial. Mol. Nutr. Food Res. 2016, 60, 1544–1554. [Google Scholar] [CrossRef] [Green Version]
- Berrougui, H.; Ikhlef, S.; Khalil, A. Extra Virgin Olive Oil Polyphenols Promote Cholesterol Efflux and Improve HDL Functionality. Evid. Based Complement Altern. Med. 2015, 2015, 208062. [Google Scholar] [CrossRef] [Green Version]
- Filipek, A.; Gierlikowska, B. Oleacein may intensify the efflux of oxLDL from human macrophages by increasing the expression of the SRB1 receptor, as well as ABCA1 and ABCG1 transporters. J. Funct. Foods 2021, 78, 104373. [Google Scholar] [CrossRef]
- Filipek, A.; Mikołajczyk, T.P.; Guzik, T.J.; Naruszewicz, M. Oleacein and Foam Cell Formation in Human Monocyte-Derived Macrophages: A Potential Strategy Against Early and Advanced Atherosclerotic Lesions. Pharmaceuticals 2020, 13, 64. [Google Scholar] [CrossRef]
- Uitz, E.; Bahadori, B.; McCarty, M.F.; Moghadasian, M.H. Practical strategies for modulating foam cell formation and behavior. World J. Clin. Cases 2014, 2, 497–506. [Google Scholar] [CrossRef]
- Filipek, A.; Czerwinska, M.E.; Kiss, A.K.; Polanski, J.A.; Naruszewicz, M. Oleacein may inhibit destabilization of carotid plaques from hypertensive patients. Impact on high mobility group protein-1. Phytomedicine 2017, 32, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; Caterina, R.D.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavall, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxid. Med. Cell Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef] [PubMed]
- Tsartsou, E.; Proutsos, N.; Castanas, E.; Kampa, M. Network Meta-Analysis of Metabolic Effects of Olive-Oil in Humans Shows the Importance of Olive Oil Consumption with Moderate Polyphenol Levels as Part of the Mediterranean Diet. Front. Nutr. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Hernáez, A.; Remaley, A.T.; Farràs, M.; Fernández-Castillejo, S.; Subirana, I.; Schröder, H.; Fernández-Mampel, M.; Muñoz-Aguayo, D.; Sampson, M.; Solà, R.; et al. Olive Oil Polyphenols Decrease LDL Concentrations and LDL Atherogenicity in Men in a Randomized Controlled Trial. J. Nutr. 2015, 145, 1692–1697. [Google Scholar] [CrossRef] [Green Version]
- Fonolla, J.; Dıaz-Ropero, P.; de la Fuente, E.; Quintela, J.C. One-month Consumption of an Olive Leaf Extract Enhances Cardiovascular Status in Hypercholesterolemic Subjects. Atheroscler. Suppl. 2010, 11, 109–222. [Google Scholar] [CrossRef]
- Hernáez, A.; Fernández-Castillejo, S.; Farràs, M.; Catalán, U.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, O.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef] [Green Version]
- Knaub, K.; Mödinger, Y.; Wilhelm, M.; Schön, C. LDL-Cholesterol Lowering Effect of Hydroxytyrosol (HTEssence®): A Randomized Double-Blind, Placebo-Controlled Parallel Study. J. Nutr. Food Sci. 2020, 10, 778. [Google Scholar] [CrossRef]
- Fonollá, J.; Maldonado-Lobón, J.A.; Luque, R.; Rodríguez, C.; Bañuelos, O.; López-Larramendi, J.L.; Olivares, M.; Blanco-Rojo, R. Effects of a Combination of Extracts from Olive Fruit and Almonds Skin on Oxidative and Inflammation Markers in Hypercholesterolemic Subjects: A Randomized Controlled Trial. J. Med. Food 2021, 24, 479–486. [Google Scholar] [CrossRef]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.J.; Fonollá, J.; Baró, L.; Bartolomé, M.V.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and miRNA Expression in Adipocytes by Attenuating NF-κB Activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; Caterina, R.D. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Aronson, D.; Rayfield, E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Gero, D. Hyperglycemia-Induced Endothelial Dysfunction. In Endothelial Dysfunction-Old Concepts and New Challenges; Lenasi, H., Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/57915 (accessed on 29 September 2022). [CrossRef]
- Anderson, R.E.; Tan, W.K.; Martin, H.S.; Meyer, F.B. Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke 1999, 30, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Yuan, S.; Zhong, Y.; Deng, L.; Li, J.; Tan, X.X.; Feng, J. Amelioration of Endothelial Dysfunction in Diabetes: Role of Takeda G Protein-Coupled Receptor 5. Front. Pharmacol. 2021, 12, 637051. [Google Scholar] [CrossRef]
- Carrillo-Sepulveda, M.A.; Maddie, N.; Johnson, C.M.; Burke, C.; Lutz, O.; Yakoub, B.; Kramer, B.; Persand, D. Vascular hyperacetylation is associated with vascular smooth muscle dysfunction in a rat model of non-obese type 2 diabetes. Mol. Med. 2022, 28, 30. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart. J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Saito, T.; Ogihara, T.; Ishigaki, Y.; Yamada, T.; Imai, J.; Uno, K.; Gao, J.; Kaneko, K.; Shimosawa, T.; et al. Blockade of the nuclear factorkappab pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 2012, 125, 1122–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgescu, A. Vascular dysfunction in diabetes: The endothelial progenitor cells as new therapeutic strategy. World J. Diabetes 2011, 2, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.E.; Lepore, S.M.; Morittu, V.M.; Arcidiacono, B.; Colica, C.; Procopio, A.; Maggisano, V.; Bulotta, S.; Costa, N.; Mignogna, C.; et al. Effects of Oleacein on High-Fat Diet-Dependent Steatosis, Weight Gain, and Insulin Resistance in Mice. Front. Endocrinol. 2018, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Jurado-Ruiz, E.; Álvarez-Amor, L.; Varela, L.M.; Berná, G.; Parra-Camacho, M.S.; Oliveras-Lopez, M.J.; Martínez-Force, E.; Rojas, A.; Hmadcha, A.; Soria, B.; et al. Extra virgin olive oil diet intervention improves insulin resistance and islet performance in diet-induced diabetes in mice. Sci. Rep. 2019, 9, 11311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive Component Oleuropein Promotes β-Cell Insulin Secretion and Protects β-Cells from Amylin Amyloid-Induced Cytotoxicity. Biochemistry 2017, 56, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, K.; Tong, T. Efficacy and Mechanisms of Oleuropein in Mitigating Diabetes and Diabetes Complications. J. Agric. Food Chem. 2021, 69, 6145–6155. [Google Scholar] [CrossRef]
- Porto, A.D.; Brosolo, G.; Casarsa, V.; Bulfone, L.; Scandolin, L.; Catena, C.; Sechi, L.A. The Pivotal Role of Oleuropein in the Anti-Diabetic Action of the Mediterranean Diet: A Concise Review. Pharmaceutics 2021, 14, 40. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Fang, J.; Geng, R.; Li, M.; Zhao, Y.; Kang, S.-G.; Huang, K.; Tong, T. Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in db/ db Mice by Regulating Gut Microbiota. Nutrients 2021, 13, 2131. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Vlavcheski, F.; Young, M.; Tsiani, E. Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence. Antioxidants 2019, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Gerrits, A.J.; Koekman, C.A.; Yildirim, C.; Nieuwland, R.; Akkerman, J.W. Insulin inhibits tissue factor expression in monocytes. J. Thromb. Haemost. 2009, 7, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Janicke, D.; Wilson, M.F.; Tripathy, D.; Garg, R.; Bandyopadhyay, A.; Calieri, J.; Hoffmeyer, D.; Syed, T.; Ghanim, H.; et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation 2004, 109, 849–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegenga, M.E.; van der Crabben, S.N.; Levi, M.; de Vos, A.F.; Tanck, M.W.; Sauerwein, H.P.; van der Poll, T. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes 2006, 55, 1807–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jotic, A.; Milicic, T.; Sternic, N.C.; Kostic, V.S.; Lalic, K.; Jeremic, V.; Mijajlovic, M.; Lukic, L.; Rajkovic, N.; Civcic, M.; et al. Decreased Insulin Sensitivity and Impaired Fibrinolytic Activity in Type 2 Diabetes Patients and Nondiabetics with Ischemic Stroke. Int. J. Endocrinol. 2015, 2015, 934791. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Gabazza, E.C.; Yano, Y.; Katsuki, A.; Suzuki, K.; Adachi, Y.; Sumida, Y. Insulin resistance is associated with increased circulating level of thrombin-activatable fibrinolysis inhibitor in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2002, 87, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Mok, M.; Christensen, A.-M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef] [Green Version]
- Petroni, A.; Blasevich, M.; Salami, M.; Papini, N.; Montedoro, G.F.; Galli, C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res. 1995, 78, 151–160. [Google Scholar] [CrossRef]
- Agrawal, K.; Melliou, E.; Li, X.; Pedersen, T.L.; Wang, S.C.; Magiatis, P.; Newman, J.W.; Holt, R.R. Oleocanthal-rich extra virgin olive oil demonstrates acute anti-platelet effects in healthy men in a randomized trial. J. Funct. Foods 2017, 36, 84–93. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; al Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, A.; Frostegard, J. Annexin A5 as a novel player in prevention of atherothrombosis in SLE and in the general population. Ann. N. Y. Acad. Sci. 2007, 1108, 96–103. [Google Scholar] [CrossRef] [PubMed]
- de Roos, B.; Zhang, X.; Gutierrez, G.R.; Wood, S.; Rucklidge, G.J.; Reid, M.D.; Duncan, G.J.; Cantlay, L.L.; Duthie, G.G.; O’Kennedy, N. Anti-platelet effects of olive oil extract: In vitro functional and proteomic studies. Eur. J. Nutr. 2011, 50, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Panzella, L.; Duarte, B.; Gonçalves-Monteiro, S.; Marques, F.; Morato, M.; Correia-da-Silva, M.; Verotta, L.; Napolitano, A. Sulfated Oligomers of Tyrosol: Toward a New Class of Bioinspired Nonsaccharidic Anticoagulants. Biomacromolecules 2021, 22, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Kezele, T.G.; Ćurko-Cofek, B. Age-Related Changes and Sex-Related Differences in Brain Iron Metabolism. Nutrients 2020, 12, 2601. [Google Scholar] [CrossRef]
- Mascitelli, L.; Pezzetta, F.; Goldstein, M.R. Is the beneficial antioxidant effect of olive oil mediated by interaction of its phenolic constituents and iron? Arch. Med. Res. 2010, 41, 295–296. [Google Scholar] [CrossRef]
- Buijsse, B.; Feskens, E.J.; Moschandreas, J.; Jansen, E.H.; Jacobs, D.R., Jr.; Kafatos, A.; Kok, F.J.; Kromhout, D. Oxidative stress, and iron and antioxidant status in elderly men: Differences between the Mediterranean south (Crete) and northern Europe (Zutphen). Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 495–500. [Google Scholar] [CrossRef]
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Virmani, R.; Finn, A.V. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine 2019, 47, 598–606. [Google Scholar] [CrossRef]
- Mascitelli, L.; Goldstein, M.R. Olive Oil-Derived Polyphenols, Iron, and Stroke Occurrence. Neurology 2011. Available online: https://n.neurology.org/content/olive-oil-derived-polyphenols-iron-and-stroke-occurrence (accessed on 29 September 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grubić Kezele, T.; Ćurko-Cofek, B. Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties. Nutrients 2022, 14, 4533. https://doi.org/10.3390/nu14214533
Grubić Kezele T, Ćurko-Cofek B. Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties. Nutrients. 2022; 14(21):4533. https://doi.org/10.3390/nu14214533
Chicago/Turabian StyleGrubić Kezele, Tanja, and Božena Ćurko-Cofek. 2022. "Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties" Nutrients 14, no. 21: 4533. https://doi.org/10.3390/nu14214533
APA StyleGrubić Kezele, T., & Ćurko-Cofek, B. (2022). Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties. Nutrients, 14(21), 4533. https://doi.org/10.3390/nu14214533