Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update
Abstract
:1. Introduction
2. Pathogenesis of Glaucoma
2.1. Mechanical Hypothesis
2.2. Vascular Hypothesis
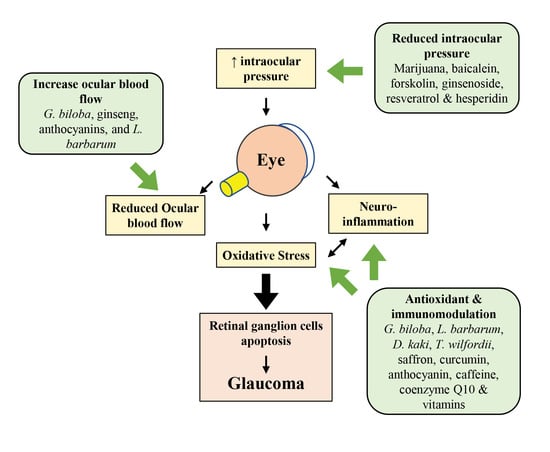
2.3. Oxidative Stress and Neuroinflammation in Glaucoma
2.4. Excitotoxicity of Glutamate
3. Glaucoma Research Models
| Research Models | Genes Involved | Mechanisms | References | |
|---|---|---|---|---|
| Genetic in vivo model | D2 mice | Tyrosinase-related protein 1 (Tyrp1) Glycoprotein non-metastatic melanoma protein B (Gpnmb) | Blockage of aqueous humor drainage, leading to progressive elevated IOP | [65] |
| Methods | Surgery involved | Mechanisms | References | |
| Experimental in vivo model | Injection | Injection of microbeads into the anterior chamber | Blockage of aqueous humor drainage, leading to elevated IOP | [70] |
| Injection of hydroxypropyl methylcellulose into the anterior chamber | Blockage of aqueous humor drainage, leading to elevated IOP | [71] | ||
| Injection of hyaluronic acid into the anterior chamber | Blockage of aqueous humor drainage, leading to elevated IOP | [72] | ||
| Injection of hypertonic saline into the episcleral vein | Produced scarring in the TM, increasing resistance to aqueous humor drainage, leading to elevated IOP | [73] | ||
| Intravitreal injection of NMDA | NMDA induced excitotoxicity, leading to RGC death | [79] | ||
| Cauterization/laser photocoagulation | Episcleral vein cauterization | Produced scarring in the TM, increasing resistance to aqueous humor drainage, leading to elevated IOP | [74] | |
| Argon laser photocoagulation of the episcleral/limbal vein | Produced scarring in the TM, increasing resistance to aqueous humor drainage, leading to elevated IOP | |||
| Nerve injury | Optic nerve crush | Optic nerve injury leading to axonal degeneration and gradual RGC loss | [80] | |
| Complete optic nerve transection | Optic nerve injury leading to axonal degeneration and gradual RGC loss | [81] | ||
| Partial optic nerve transection | Optic nerve injury leading to axonal degeneration and gradual RGC loss | [83] | ||
| Retinal I/R injury | Reduced retinal blood flow by induction of elevated IOP (ischemia), followed by reinstation of blood flow (reperfusion) | Extreme acute OHT-induced ischemic injury to RGC, followed by severe oxidative and inflammatory damage to RGCs after reperfusion | [84,85] |
4. Natural Products Used for Glaucoma Treatment and Their Mechanism of Action
4.1. Gingko biloba L.
4.2. Scutellaria baicalensis Georgi—Baicalin, Baicalein and Wogonin
4.3. Coleus forskohlii (willd.) Briq.—Forskolin
4.4. Erigeron breviscapus (vant.) Hand. Mazz.—Scutellarin
4.5. Lycium barbarum L.
4.6. Diospyros kaki L.
4.7. Tripterygium wilfordii Hook F.—Triptolide and Celastrol
4.8. Crocus sativus L.—Crocetin and Crocin
4.9. Curcuma longa L.—Curcumin
4.10. Camellia sinensis (L.) Kuntze—Epigallocatechin-3-Gallate
4.11. Panax ginseng—Ginsenoside
4.12. Cannabis sativa—Cannabinoids
4.13. Anthocyanins
4.14. Resveratrol
4.15. Hesperidin
4.16. Caffeine
4.17. Coenzyme Q10
4.18. Vitamins
| Natural Products | Subjects | Treatment Regime | Clinical Findings | References |
|---|---|---|---|---|
| Ginkgo biloba | POAG patients | 120 mg GB extract, 1 tablet daily, 6 months | Lower rate of single-stranded DNA breaks in circulating leukocytes (vs. untreated patients, p < 0.001) | [97] |
| NTG patients | 80 mg GB extract, 2 tablets daily, 4 years | No effect on IOP (vs. pre-treatment, p = 0.509) Slowed visual field damage progression (p < 0.001) | [98] | |
| NTG patients | 80 mg GB extract, 2 tablets daily, 2 years | Improved HVF deviation (vs. untreated patients, p = 0.002) | [99] | |
| NTG patients | 80 mg GB extract, 2 tablets daily, 4 weeks | Increased ocular blood flow, volume and velocity (vs. placebo-treated patients, p < 0.03) | [100] | |
| Healthy subjects | 120 mg GB extract, 1 tablet daily, 4 weeks | Increased radial peripapillary capillary vascular density (vs. pre-treatment, p < 0.021) | [101] | |
| Forskolin | POAG patients | Forskolin 1% w/v aqueous solution eye drops, 2 drops thrice a day, 4 weeks | Reduced IOP (vs. timolol-treated patients, p < 0.05) No adverse events | [117] |
| Erigeron breviscapus | POAG patients | E. breviscapus extract, 2 tablets, 3 times daily, 6 months | No obvious adverse effects Decreased mean defect (vs. pre-treatment, p < 0.01) Increased mean sensitivity (p < 0.01) | [122] |
| Saffron | POAG patients | Aqueous saffron extract, 30 mg daily, 4 weeks | Reduced IOP (vs. pre-treatment, p = 0.0046) No obvious adverse effects | [160] |
| Ginseng | Glaucoma patients | Korean red ginseng, 3 g daily, 4 weeks | Improved daytime contrast sensitivity (vs. pre-treatment, p = 0.004) and ocular pain (p < 0.001) | [186] |
| Glaucoma patients | Korean red ginseng, 3 g daily, 8 weeks | Improved tear film stability and total OSDI score (vs. placebo-treated patients, p < 0.01) | [187] | |
| OAG patients | Korean red ginseng, 1.5 g, 3 times daily, 12 weeks | Improved retinal peripapillary blood flow in the temporal peripapillary region (vs. pre-treatment, p = 0.005) No changes in blood pressure, heart rate, IOP and visual field indices | [188] | |
| Marijuana | Glaucoma patients | Marijuana smoking, single dose | Reduced IOP (vs. placebo-treated patients, p value not defined) Increased heart rate | [195] |
| Healthy subjects | Marijuana smoking, single dose | Reduced IOP (vs. pre-treatment, p < 0.01) No effect on systemic blood pressure | [196] | |
| Anthocyanins | NTG patients | 60 mg, 2 tablets daily, 2 years | Improved best-corrected visual acuity (vs. untreated patients, p = 0.008), and HVF deviation (p = 0.001) | [99] |
| OAG patients | 50 mg black currant anthocyanins daily, 2 years | Increased ocular blood flows (vs. placebo-treated patients, p = 0.01) Improved visual field damage progression (p = 0.039) | [204] | |
| OAG patients | 50 mg black currant anthocyanins daily, 24 months | Reduced IOP (vs. pre-treatment, p = 0.027) Improved HVF deviation (p = 0.017) No changes in systemic blood pressure or pulse rates | [205] | |
| OAG patients | 50 mg black currant anthocyanins daily, 24 months | Normalized serum ET-1 concentrations (vs. healthy subjects, p < 0.05) No changes in advanced oxidation protein products, and antioxidative activities | [206] | |
| Hesperidin, crocetin and Tamarindus indica | NTG patients | Food supplement containing hesperidin (50 mg), crocetin (7.5 mg) and T. indica (25 mg), 4 tablets twice a day, 8 weeks | Reduced 8-OHdG level in high-oxidative stress patients (vs. pre-treatment, p < 0.01) Elevated BAP in high-oxidative stress patients (p = 0.03) | [222] |
| Caffeine | POAG patients | Coffee containing 1.3% caffeine (104 mg caffeine), single dose | Reduced IOP (vs. water-drinking patients, p = 0.012) Reduced IOP fluctuation (p = 0.013) | [228] |
| POAG patients | 1% caffeine eye drop, thrice a day, 1 week | No effect on IOP (vs. pre-treatment, p > 0.05) | [229] | |
| Healthy subjects | Caffeine capsule, 4 mg/kg, single dose | Increased IOP (vs. pre-treatment, p < 0.05) | [230] | |
| Healthy subjects | Caffeine capsule, 4 mg/kg, single dose | Increased IOP (vs. placebo-treated subjects, p < 0.05) Reduced anterior chamber angle (p < 0.05) | [231] | |
| Coenzyme Q10 | POAG patients | CoQ10 and vitamin E eye drop, 2 drops daily, 12 months | Decreased ERG P50 and VEP P100 implicit times (vs. pre-treatment, p < 0.01) Increased PERG P50-N95 and VEP N75-P100 amplitudes (p < 0.01) | [242] |
| Vitamin B3 | Glaucoma patients | Vitamin B3 tablet, 1.5 g/day 6 weeks, followed by 3.0 g/day for 6 weeks | Improved RGC functions—PhNR Vmax (vs. placebo-treated patients, p = 0.03), Vmax ratio (p = 0.02) and visual field mean deviation (p = 0.02) No effect on IOP (p = 0.59) and RNFL thickness (p = 0.11) | [249] |
| Natural Products | Model | RGC | IOP | Ocular Vasculation | Other Findings | References |
|---|---|---|---|---|---|---|
| Ginkgo biloba | Rat RGC cells exposed to H2O2 | Increased survival rate | - | - | - | [96] |
| Rat optic nerve crush model | Increased RGC density | - | - | - | [96] | |
| Rat optic nerve crush model | Increased survival rate | - | - | - | [102] | |
| Mouse RGC-5 cells exposed to H2O2 | Reduced cell apoptosis | - | - | Increased antioxidant capacity (reduced T-AOC, SOD and CAT depletion) | [105] | |
| Diterpene ginkgolides meglumine injection | Rat optic nerve injury model | Reduced cell apoptosis | - | - | Decreased conduction time of F-VEP | [103] |
| Scutellaria baicalensis—Baicalein | Rat episcleral vein cauterization-induced chronic OHT model | - | Reduced IOP | - | - | [109] |
| Rat ischemic model | Reduced cell apoptosis | - | - | Upregulation of HO-1 Downregulation of HIF-1α, VEGF and MMP-9 | [110] | |
| S. baicalensis—Wogonin | Rat optic nerve crush model | Reduced cell apoptosis | - | - | Decreased caspase-3 activation Decreased gliosis response and microglial activation Decreased pro-inflammatory cytokine (TNF-α, MCP-1, iNOS, IL-6 and-1β and COX-2) expression | [111] |
| S. baicalensis—Baicalin | NMDA-stimulated RGC | Reduced cell apoptosis | - | - | Alleviated NMDA-induced oxidative stress (reduced ROS and MDA levels) Inhibited NMDA-induced autophagy | [112] |
| Mouse episcleral venous occlusion- induced chronic OHT model | Increased RGC density Increased GCL thickness | - | - | Inhibited OHT-induced autophagy Activated PI3K/AKT signaling | [112] | |
| Forskolin | Isolated bovine eye | - | Reduced IOP | - | Reduced peak calcium response to ATP | [116] |
| Forskolin, homotaurine, spearmint extract and vitamins B1, B2 and B12 mixture | Mouse optic nerve crush model | Increased RGC numbers | - | - | Reduced cytokine (iNOS and IL-6) secretion Decreased apoptotic marker (Bax/Bcl-2 ratio and active caspase-3) levels | [119] |
| Rat methylcellulose-induced OHT model | Increased RGC numbers | No effect | - | Prevented the reduction in retinal function (increased PhNR amplitude, PERG amplitude and implicit time) Prevented microglial and Müller cell activation Decreased inflammatory markers (NF-κB, TNF-α and IL-6) Decreased apoptotic marker (Bax/Bcl-2 ratio and active caspase-3) levels | [120] | |
| Sodium alginate poly (vinyl alcohol) electrospun nanofibers of forskolin | Normal rabbit | - | Reduced IOP | - | - | [257] |
| Erigeron breviscapus | Rat episcleral vein cauterization-induced OHT model | - | Reduced IOP | - | Improved visual function | [123] |
| Rabbit methylcellulose-induced OHT model | Increased RGC density Increased RNFL thickness Reduced RGC axonal degeneration | - | - | - | [124] | |
| Scutellarin | Mouse clear hydrogel-induced OHT model | - | - | - | Reduced retinal thinning Reduced visual behavioral deficits | [126] |
| BV-2 cells exposed to low oxygen level | - | - | - | Increased cell viability Inhibited expression of NLRP3 Reduced the upregulation of ASC, cleaved caspase-1 and IL-18 and -1β | [127] | |
| Rat saline-induced acute OHT model | Increased survival rate | - | - | Reduced impaired microglial cells Inhibited NLRP3 expression Reduced upregulation of ASC, cleaved caspase-1 and IL-18 and -1β | [127] | |
| Lycium barbarum | Rat argon laser photocoagulation-induced OHT model | Reduced ET-1 expression in RGCs | - | - | - | [131] |
| Mouse acute OHT model | Increased RGC numbers Increased IRL thickness | - | Recovered blood vessel density in retina | Protected retinal vasculature stability (reduced IgG leakage, more continued structure of tight junctions associated with increased occludin protein level) Downregulation of RAGE, ET-1, Aβ and AGE | [131] | |
| Rat acute OHT model | Normalized GCL density Preserved IRL thickness | - | - | Preserved positive scotopic threshold response functions | [132] | |
| Rat suture implantation-induced chronic OHT model | Preserved RGCs | - | - | - | [134] | |
| Rat partial optic nerve transection model | - | - | - | Preserved visual function | [135] | |
| Rat complete and partial optic nerve transection | Delayed RGC degeneration | - | - | Increased MnSOD and IGF-1 expressions | [136] | |
| RGC-5 cells exposed to CoCl2-induced hypoxia | Reduced cell apoptosis | - | - | Inhibited ROS generation Inhibited reduction in mitochondrial membrane potential | [137] | |
| Human TM cells exposed to H2O2 | - | - | - | Promoted cell viability Reduced apoptosis Reduced cleaved caspase-3/-9 and ROS levels | [138] | |
| Rat partial optic nerve transection model | Delayed secondary degeneration of RGCs | - | - | Promoted M2 polarization of microglia/macrophages Downregulated autophagy level | [139] | |
| PC12 cells exposed to hydrostatic pressures | - | - | - | Reduced ANGPTL7, MMP-2 and -9, collagen I and TGF-β expressions | [141] | |
| Mouse retinal I/R injury model | Retinal cellular organization remained normal Fewer pyknotic nuclei in GCL and INL | - | - | Reduced glial activation | [144] | |
| Rat retinal I/R injury model | Reduced apoptosis in GCL and INL | - | - | Increased Nrf2 nuclear accumulation Increased HO-1 expression | [145] | |
| Rat saline-induced acute OHT model | Downregulation of APP and RAGE expressions | - | Reverse loss of function of astrocyte endfeet around blood vessels | Reduced numbers of astrocytes and microglia Decreased glutamine toxicity in astrocytes (downregulation of glutamine synthetase) | [146] | |
| Rat retinal I/R injury model | - | - | - | Preserved retinal thickness Increased antioxidant levels (GSSH + GSH, SOD and CAT) Reduced MDA level | [146] | |
| Diospyros kaki | Mouse microbead-induced OHT model, and D2 mouse | Reduced RGC loss | Reduced IOP | - | Increased sGCα-1 expression | [149] |
| RGC-5 cells exposed to glutamate | Increased cell viability | - | - | Decreased apoptotic protein levels (poly (ADP-ribose) polymerase, p53 and cleaved caspase-3) Increased antioxidant-associated protein expression levels (SOD, GST and GPX) | [150] | |
| Mouse partial optic nerve crush model | Reduced RGC death | - | - | - | [150] | |
| T. wilfordii—Triptolide | D2 mouse | Improved RGC survival | No effect | - | Suppressed microglia activation | [153] |
| Angle photocoagulation-induced chronic glaucoma rat model | Improved RGC survival | - | - | Reduced microglia count Reduced TNF-α expression | [154] | |
| Mouse optic nerve crush model | Improved RGC survival | - | - | Reduced TNF-α expression Inhibited nuclear translocation of NF-κB | [155] | |
| T. wilfordii—celastrol | Mouse optic nerve crush model | Improved RGC survival | - | - | Reduced TNF-α expression | [156] |
| Rat trabecular laser photocoagulation model | Improved RGC survival | - | - | - | [157] | |
| Crocus sativus L. | Mouse laser-induced OHT model | Prevented RGC death | - | - | Decreased microglial numbers and their activation Partially reversed downregulation of P2RY12 | [159] |
| C. sativus—Crocin | Rat retinal I/R injury model | Increased RGC survival | - | - | Inhibited retinal thinning Decreased cleaved caspase-3 and p-ERK protein expressions Increased GSH and T-SOD activities Decreased ROS and MDA levels | [162] |
| Rat retinal I/R injury model | Increased RGC survival Reduced RGC apoptosis | - | - | Upregulation of Bcl-2/Bax level Enhanced p-AKT levels | [163] | |
| RGC-5 cells exposed to H2O2 | Protected RGCs from apoptosis Enhanced cell viability | - | - | Decreased LDH release Decreased ROS levels Increased ΔΨm Downregulated Bax and cytochrome c protein expressions Promoted Bcl-2 protein expression Activated NF-κB | [164] | |
| C. sativus—Crocetin | Mouse NMDA-induced retinal injury model | Increased GCL density | - | - | Reduced TUNEL-positive cells Inhibited activated caspase-3/-7 Increased cleaved caspsase-3 expression | [165] |
| Rat retinal I/R injury model | Increased GCL density Reduced INL thinning | - | - | Decreased TUNEL-positive cells and 8-OHdG-positive cells Decreased phosphorylation levels of p38, JNK, NF-κB and c-Jun | [166] | |
| Curcumin | BV-2 cells exposed to H2O2 | - | - | - | Increased cell viability Decreased ROS and apoptosis Downregulated caspase-3, cytochrome c and Bax Upregulated Bcl-2 | [169] |
| Rat episcleral vein cauterization | Prevented RGC loss | - | - | Downregulated caspase-3, cytochrome c and Bax Upregulated Bcl-2 | [169] | |
| Ex vivo optic nerve cut model | Increased RGC survival Preserved retinal thickness | Prevented alterations in apoptotic cascades and MAPK and SUMO-1 pathways | [170] | |||
| Rat retinal I/R injury model | - | - | - | Prevented retinal damage | [171] | |
| Rat retinal I/R injury model | Inhibited GCL cell loss Reduced cell apoptosis | Inhibited retinal capillary degeneration Inhibited upregulation of MCP-1, IKKα, p-IκBα and p-STAT3 (Tyr), and downregulation of β-tubulin II | [172] | |||
| Primary porcine TM cells exposed to H2O2 | - | - | - | Prevented cell death Reduced ROS production Inhibited pro-inflammatory factors (IL-6, -1α and -8 and ELAM-1) Decreased SA-β-gal activity Reduced carbonylated proteins and apoptotic cell numbers | [173] | |
| Primary porcine TM cells exposed to H2O2 | - | - | - | Reduced ROS level Reduced apoptosis Upregulated Bcl-2 Downregulated Bax and activated caspase-3 levels Reduced Nrf2, HO-1 and NQO1 expressions Increased Keap1 expression | [174] | |
| Rat partial optic nerve transection model | Improved RGC density ratio | No effect | - | - | [258] | |
| Human TM cells exposed to H2O2 | - | - | - | Reduced TNF and IL-1α and -6 expression Reduced mitochondrial ROS production Reduced cleaved caspase-3 proteins Reduced TUNEL-positive cells | [259] | |
| Green tea | Rat retinal I/R injury model | Increased RGC numbers Reduced apoptotic RGCs | - | - | Reduced activated caspase-3 and -8, SOD2 and inflammation-related proteins expressions Reduced p38 phosphorylation Enhanced JAK phosphorylation | [176] |
| Rat LPS-induced retinal inflammation model | - | - | - | Suppressed activated microglia, astrocytes and Müller glia Reduced pro-inflammatory cytokine expressions (IL-1β and -6 and TNF-α in retina and vitreous humor) | [177] | |
| Green tea—EGCG | Rat saline-induced acute OHT model | - | - | - | Decreased inflammation-associated cytokine levels Decreased the proliferation rate of T lymphocyte cells Reduced IκBα and p65 phosphorylation | [179] |
| Mouse microbead-induced OHT model | Increased RGC numbers | No effect | - | - | [180] | |
| Rat optic nerve crush model | Increase RGC density | - | - | Increased NF-L protein expression | [181] | |
| Rabbit retinal I/R injury model | Preserved organization of GCL, IPL and INL | - | - | Reduced retinal gliosis Reduced MDA level | [182] | |
| Rat NMDA-induced excitotoxicity model | Increased GCL cell density | - | - | - | [183] | |
| Ginseng | Rat optic nerve crush injury model | Increased cell survival Reduced cell apoptosis | - | - | Increased Bcl-2/Bax protein ratio Decreased c-Jun, P-c-Jun and P-JNK protein expressions | [190] |
| Rabbit ultrasound-targeted microbubble optic nerve injury model | Reduced RGC damage | Reduced IOP | - | Reduced oxidative stress level Reduced MDA and NO levels Increased SOD level | [191] | |
| RGC-5 cells exposed to CoCl2 or H2O2 | Reduced cell apoptosis | - | - | Reduced cleaved caspase-3 and -9 expressions | [192] | |
| Marijuana—Δ9-THC | Normal dogs | - | Reduced IOP | - | No effect on aqueous humor flow rate | [197] |
| Normal rabbit | - | Reduced IOP | - | - | [198] | |
| Marijuana—Δ8-THC | Rabbit chymotrypsin-induced OHT model | - | Reduced IOP | - | - | [199] |
| Marijuana | Rat retinal I/R injury model | Reduced RGC damage | - | - | - | [201] |
| Anthocyanins | RGC-5 cells exposed to H2O2 | Increased survival rate | - | - | - | [207] |
| Mouse optic nerve crush model | Increased survival rate | - | - | Increased Grp78 and Grp94 levels | [208] | |
| Resveratrol | Glaucomatous human TM cells | - | - | - | Increased eNOS and NO levels Decreased iNOS expressions Increased IL-1α level with low dose Decreased IL-1α level with high dose | [211] |
| Rat hyaluronic acid-induced chronic OHT model | Preserved RGC numbers | No effect | - | - | [212] | |
| Mouse microbead-induced OHT model | Preserved RGC numbers | - | - | Decreased ROS generation and acetyl-p53 expression Upregulated BDNF and TrkB expressions | [213] | |
| RGC-5 cells exposed to H2O2 | Increased cell viability | - | - | Reduced expressions of cleaved caspase-3 and -9 Reduced ROS production Reduced loss of mitochondrial membrane potential and p-p38, p-ERK and p-JNK expressions Promoted SOD, CAT and GSH activities | [214] | |
| Mouse retinal I/R injury model | Ameliorated retinal thickness damage Increased RGC numbers | - | - | Downregulated mitochondrial apoptosis-related proteins (Bax and cleaved caspase-3) Increased Bcl-2 expression | [215] | |
| Mouse retinal I/R injury model | Reduced RGC loss Reduced retinal damage | - | - | Reduced TUNEL staining Reduced Bax and cleaved caspase-3 levels | [216] | |
| Mouse retinal I/R injury model | Reduced RGC loss | - | - | Reduced Bcl-2, Bax, caspase-3, GFAP, COX-2 and iNOS expressions | [217] | |
| Rat superparamagnetic iron oxide-induced chronic OHT model | No effect on GCL density Decreased cell apoptosis | No effect | - | Improved retinal morphology Improved expressions of proteins involved in mitochondrial biogenesis and dynamics | [218] | |
| RGC-5 cells exposed to elevated pressure | Decreased cell apoptosis | - | - | Decreased mitochondrial membrane potential depolarization Decreased ROS production Upregulated expressions of proteins involved in mitochondrial biogenesis and dynamics | [218] | |
| Mouse retinal I/R injury model | Decreased cell apoptosis Restored retina thickness | Increased Opa1 expression, and long Opa1 isoform-to-short Opa1 isoform ratios | [219] | |||
| Normal rabbit | - | Reduced IOP | - | - | [260] | |
| Hesperidin | Rat dextrose- or prednisolone acetate-induced OHT model | - | Reduced IOP | - | Increased glutathione Reduced morphological alteration in ciliary bodies | [223] |
| Mouse NMDA-induced retinal injury model | - | - | - | Reduced inflammatory cytokine (TNF-α, IL-1b and -6 and MCP-1) expressions | [224] | |
| Mouse NMDA-induced retinal injury model | Prevented reductions in RGC markers Prevented RGC death | - | - | Reduced calpain activation, ROS generation and TNF-α gene expression Improved electrophysiological function and visual function | [225] | |
| Rat hypobaric hypoxia-induced retinal injury model | - | - | - | Enhanced Nrf2 and HO-1 activation Attenuated apoptotic caspase levels Reduced Bax and preserved Bcl-2 expressions Downregulated PARP1 expression Upregulated CNTF expression | [226] | |
| Caffeine | Rat laser-induced OHT model | Increased survival rate | Reduced IOP | - | Downregulated TNF and IL-1β mRNA and protein levels Suppressed microglia activation (downregulated MHC-II, TSPO, CD11b and TREM2 expressions) | [234] |
| Rat retinal I/R injury model | - | - | - | Reduced microglial activation at 7 days post-injury (reduced Iba1 and MHC-II cells; reduced TSPO and MHC-II mRNA levels) Reduced TUNEL-positive cells | [235] | |
| Human retinal pigment epithelial cells exposed to LPS | - | - | - | Reduced LPS-induced inflammatory cytokines (TNF-α, IL-1β and -6) Restored BDNF expression Reduced p-NF-κB p65 nuclear translocation Restored blood–retinal barrier (increased transepithelial electrical resistance value and ZO-1 tight junction expression) | [236] | |
| Mouse retinal I/R injury model | - | - | - | Increased PERG amplitude Reduced IL-6 mRNA expression Increased BDNF mRNA expression | [236] | |
| Coenzyme Q10 | Mouse retinal ischemia model | Promoted RGC survival | - | - | Prevented upregulation of SOD2 and HO-1 protein expression Blocked activation of astrocytes and microglial cells Blocked apoptosis by decreasing caspase-3 protein expression Decreased Bax protein expression Preserved Tfam protein expression | [239] |
| D2-Gpnmb+ mice | Promoted RGC survival | - | - | Preserved axons in the ONH Inhibited astrocytes activation Blocked the upregulation of NR1, NR2A, SOD2 and HO1 protein expressions Decreased Bax protein expression Preserved mtDNA content and Tfam/OXPHOS complex IV protein expressions | [240] | |
| Rat chronic OHT model | Prevented RGC apoptosis and RGC loss | No effect | - | - | [241] | |
| Rat mechanic optic nerve injury model | Increased RGC numbers | - | - | Reduced activation of astroglia and microglial cells Increased Bcl-xL protein expression | [243] | |
| Vitamin B3 | D2-Gpnmb+ mouse | Prevented RGC loss Prevented RNFL thinning | Reduced IOP at high dose | - | Prevented the decline in NAD levels Reduced incidence of optic nerve degeneration Improved PERG amplitude Inhibited formation of dysfunctional mitochondria Decreased PARP activation Reduced DNA damage Reduced HIF-1α transcriptional induction | [247] |
| D2 mouse | Increased RGC density | - | - | Increased F-PERG adaptation | [248] | |
| Vitamin D | Normal monkeys | - | Reduced IOP | - | - | [253] |
| D2 mouse | Reduced RGC death | - | - | Improved PERG and FERG amplitudes Increased neuroprotective factor (BDNF, VEGF-A and PlGF) mRNA levels Decreased microglial and astrocyte activation Decreased inflammatory cytokine (IL-1β, -6, IFN-γ and CCL-3) expressions Decreased NF-κB activation | [254] | |
| Vitamin E | Rat episcleral vein cauterization | No effect | No effect | - | Increased serum vitamin E level | [255] |
| Rat optic nerve crush model | Preserved RGC numbers | - | - | - | [256] |
5. Challenges for Natural Product Application in Glaucoma Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bourne, R.R.; Taylor, H.R.; Flaxman, S.R.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; White, R.A.; Wong, T.Y.; Resnikoff, S.; et al. Number of people blind or visually impaired by glaucoma worldwide and in world regions 1990–2010: A meta-analysis. PLoS ONE 2016, 11, e0162229. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019; pp. 1–160. [Google Scholar]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Bajwa, M.N.; Malik, M.I.; Siddiqui, S.A.; Dengel, A.; Shafait, F.; Neumeier, W.; Ahmed, S. Two-stage framework for optic disc localization and glaucoma classification in retinal fundus images using deep learning. BMC Med. Inform. Decis. Mak. 2019, 19, 136. [Google Scholar] [CrossRef]
- Tharmathurai, S.; Muhammad-Ikmal, M.K.; Razak, A.A.; Che-Hamzah, J.; Azhany, Y.; Fazilawati, Q.; Liza-Sharmini, A.T. Depression and severity of glaucoma among older adults in urban and suburban areas. J. Glaucoma 2021, 30, e205–e212. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, H.; Wan Abdul Halim, W.H.; Azizi, H.A.; Hing, S.T.; Zainal Rain, S.L.; Abdul Rahman, G.Y.; Mohd Khialdin, S. Quality of life and severity of glaucoma: A study using Glaucol-36 Questionnaire at Universiti Kebangsaan Malaysia Medical Centre (UKMMC). Int. Med. J. 2017, 24, 61–64. [Google Scholar]
- Ko, F.; Boland, M.V.; Gupta, P.; Gadkaree, S.K.; Vitale, S.; Guallar, E.; Zhao, D.; Friedman, D.S. Diabetes, triglyceride levels, and other risk factors for glaucoma in the National Health and Nutrition Examination Survey 2005–2008. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Kreft, D.; Doblhammer, G.; Guthoff, R.F.; Frech, S. Prevalence, incidence, and risk factors of primary open-angle glaucoma—A cohort study based on longitudinal data from a German public health insurance. BMC Public Health 2019, 19, 851. [Google Scholar] [CrossRef]
- Chen, M.; Yu, X.; Xu, J.; Ma, J.; Chen, X.; Chen, B.; Gu, Y.; Wang, K. Association of gene polymorphisms with primary open angle glaucoma: A systematic review and meta-analysis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Souzeau, E.; Ong, J.S.; An, J.; Siggs, O.M.; Burdon, K.P.; Best, S.; Goldberg, I.; Healey, P.R.; Graham, S.L.; et al. Myocilin gene Gln368Ter variant penetrance and association with glaucoma in population-based and registry-based studies. JAMA Ophthalmol. 2019, 137, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Choplin, N.T. Classification of glaucoma. In Atlas of Glaucoma; Choplin, N.T., Traverso, C.E., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 7–12. [Google Scholar]
- Khaled Alsirhani, E.; Sahli Abdulaziz Ali, Y.; Mutlaq Ayidh Alosaimi, S.; Ahmed Ali Alkhawajah, S.; Khalifah Alsaqer, S.; Alanazi, M.S.H.; Alanzi, H.O.H.; Alghamdi, L.S.A.; Salman Alfaifi, A.; Almutairi, J.A. An overview of glaucoma diagnosis & management: A literature review. Arch. Pharm. Pract. 2020, 11, 66–69. [Google Scholar]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Tawfik, M.A.; Waisbourd, M.; Katz, L.J. Primary angle-closure glaucoma: An update. Acta Ophthalmol. 2016, 94, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Standring, S., Ed.; Elsevier Limited: New York, NY, USA, 2020; pp. 1–1606. [Google Scholar]
- Li, L.; Song, F. Biomechanical research into lamina cribrosa in glaucoma. Natl. Sci. Rev. 2020, 7, 1277–1279. [Google Scholar] [CrossRef]
- Irnaten, M.; Zhdanov, A.; Brennan, D.; Crotty, T.; Clark, A.; Papkovsky, D.; O’Brien, C. Activation of the NFAT-calcium signaling pathway in human lamina cribrosa cells in glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhavoronkov, A.; Izumchenko, E.; Kanherkar, R.R.; Teka, M.; Cantor, C.; Manaye, K.; Sidransky, D.; West, M.D.; Makarev, E.; Csoka, A.B. Pro-fibrotic pathway activation in trabecular meshwork and lamina cribrosa is the main driving force of glaucoma. Cell Cycle 2016, 15, 1643–1652. [Google Scholar] [CrossRef]
- Ivers, K.M.; Sredar, N.; Patel, N.B.; Rajagopalan, L.; Queener, H.M.; Twa, M.D.; Harwerth, R.S.; Porter, J. In Vivo changes in lamina cribrosa microarchitecture and optic nerve head structure in early experimental glaucoma. PLoS ONE 2015, 10, e0134223. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Li, J.; Fan, X.; Lin, C.; Wang, N. The influence of different intraocular pressure on lamina cribrosa parameters in glaucoma and the relation clinical implication. Sci. Rep. 2021, 11, 9755. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, T.W.; Weinreb, R.N.; Lee, E.J.; Girard, M.J.A.; Mari, J.M. Lamina cribrosa morphology predicts progressive retinal nerve fiber layer loss in eyes with suspected glaucoma. Sci. Rep. 2018, 8, 738. [Google Scholar] [CrossRef] [Green Version]
- Maddineni, P.; Kasetti, R.B.; Patel, P.D.; Millar, J.C.; Kiehlbauch, C.; Clark, A.F.; Zode, G.S. CNS axonal degeneration and transport deficits at the optic nerve head precede structural and functional loss of retinal ganglion cells in a mouse model of glaucoma. Mol. Neurodegener. 2020, 15, 48. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Ferguson, T.J.; Samuelson, T.W. Periodic normalization of the translaminar pressure gradient prevents glaucomatous damage. Med. Hypotheses 2020, 144, 110258. [Google Scholar] [CrossRef]
- Li, L.; Bian, A.; Cheng, G.; Zhou, Q. Posterior displacement of the lamina cribrosa in normal-tension and high-tension glaucoma. Acta Ophthalmol. 2016, 94, e492–e500. [Google Scholar] [CrossRef]
- Pircher, A.; Remonda, L.; Weinreb, R.N.; Killer, H.E. Translaminar pressure in Caucasian normal tension glaucoma patients. Acta Ophthalmol. 2017, 95, e524–e531. [Google Scholar] [CrossRef] [Green Version]
- Siaudvytyte, L.; Januleviciene, I.; Ragauskas, A.; Bartusis, L.; Meiliuniene, I.; Siesky, B.; Harris, A. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J. Ophthalmol. 2014, 2014, 937360. [Google Scholar] [CrossRef]
- Trivli, A.; Koliarakis, I.; Terzidou, C.; Goulielmos, G.N.; Siganos, C.S.; Spandidos, D.A.; Dalianis, G.; Detorakis, E.T. Normal-tension glaucoma: Pathogenesis and genetics. Exp. Ther. Med. 2019, 17, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Burgoyne, C.F. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp. Eye Res. 2011, 93, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Shiga, Y.; Kunikata, H.; Aizawa, N.; Kiyota, N.; Maiya, Y.; Yokoyama, Y.; Omodaka, K.; Takahashi, H.; Yasui, T.; Kato, K.; et al. Optic nerve head blood flow, as measured by laser speckle flowgraphy, is significantly reduced in preperimetric glaucoma. Curr. Eye Res. 2016, 41, 1447–1453. [Google Scholar] [CrossRef]
- Shiga, Y.; Aizawa, N.; Tsuda, S.; Yokoyama, Y.; Omodaka, K.; Kunikata, H.; Yasui, T.; Kato, K.; Kurashima, H.; Miyamoto, E.; et al. Preperimetric glaucoma prospective study (PPGPS): Predicting visual field progression with basal optic nerve head blood flow in normotensive PPG eyes. Transl. Vis. Sci. Technol. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Cai, Y.; Li, M.; Chen, X.; Kang, L.; Yang, L. Relationship between nailfold capillary morphology and retinal thickness and retinal vessel density in primary open-angle and angle-closure glaucoma. Acta Ophthalmol. 2020, 98, e882–e887. [Google Scholar] [CrossRef] [PubMed]
- Abegao Pinto, L.; Willekens, K.; Van Keer, K.; Shibesh, A.; Molenberghs, G.; Vandewalle, E.; Stalmans, I. Ocular blood flow in glaucoma—The Leuven Eye Study. Acta Ophthalmol. 2016, 94, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Tobe, L.A.; Harris, A.; Hussain, R.M.; Eckert, G.; Huck, A.; Park, J.; Egan, P.; Kim, N.J.; Siesky, B. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br. J. Ophthalmol. 2015, 99, 609–612. [Google Scholar] [CrossRef]
- Kiyota, N.; Shiga, Y.; Omodaka, K.; Pak, K.; Nakazawa, T. Time-course changes in optic nerve head blood flow and retinal nerve fiber layer thickness in eyes with open-angle glaucoma. Ophthalmology 2021, 128, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S. Controversies in the vascular theory of glaucomatous optic nerve degeneration. Taiwan J. Ophthalmol. 2016, 6, 182–186. [Google Scholar] [CrossRef]
- Chidlow, G.; Wood, J.P.M.; Casson, R.J. Investigations into hypoxia and oxidative stress at the optic nerve head in a rat model of glaucoma. Front. Neurosci. 2017, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Jassim, A.H.; Fan, Y.; Pappenhagen, N.; Nsiah, N.Y.; Inman, D.M. Oxidative stress and hypoxia modify mitochondrial homeostasis during glaucoma. Antioxid. Redox Signal. 2021, 35, 1341–1357. [Google Scholar] [CrossRef]
- Hondur, G.; Goktas, E.; Yang, X.; Al-Aswad, L.; Auran, J.D.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M.; Suh, L.H.; Trief, D.; et al. Oxidative stress-related molecular biomarker candidates for glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4078–4088. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Shao, M.; Li, Y.; Li, X.; Wan, Y.; Sun, X.; Cao, W. Relationship between oxidative stress biomarkers and visual field progression in patients with primary angle closure glaucoma. Oxidative Med. Cell. Longev. 2020, 2020, 2701539. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, S.; Cao, W.; Sun, X. The association of oxidative stress status with open-angle glaucoma and exfoliation glaucoma: A systematic review and meta-analysis. J. Ophthalmol. 2019, 2019, 1803619. [Google Scholar] [CrossRef] [Green Version]
- Margeta, M.A.; Lad, E.M.; Proia, A.D. CD163+ macrophages infiltrate axon bundles of postmortem optic nerves with glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2449–2456. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Han, J.; Cao, W.; Sun, X. Associations between blood cell profiles and primary open-angle glaucoma: A retrospective case-control study. Ophthalmic Res. 2020, 63, 413–422. [Google Scholar] [CrossRef]
- Huang, P.; Qi, Y.; Xu, Y.S.; Liu, J.; Liao, D.; Zhang, S.S.; Zhang, C. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J. Glaucoma 2010, 19, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kondkar, A.A.; Azad, T.A.; Almobarak, F.A.; Kalantan, H.; Al-Obeidan, S.A.; Abu-Amero, K.K. Elevated levels of plasma tumor necrosis factor alpha in patients with pseudoexfoliation glaucoma. Clin. Ophthalmol. 2018, 12, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Kondkar, A.A.; Sultan, T.; Almobarak, F.A.; Kalantan, H.; Al-Obeidan, S.A.; Abu-Amero, K.K. Association of increased levels of plasma tumor necrosis factor alpha with primary open-angle glaucoma. Clin. Ophthalmol. 2018, 12, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Duvesh, R.; Puthuran, G.; Srinivasan, K.; Rengaraj, V.; Krishnadas, S.R.; Rajendrababu, S.; Balakrishnan, V.; Ramulu, P.; Sundaresan, P. Multiplex cytokine analysis of aqueous humor from the patients with chronic primary angle closure glaucoma. Curr. Eye Res. 2017, 42, 1608–1613. [Google Scholar] [CrossRef]
- Wang, L.; Cioffi, G.A.; Cull, G.; Dong, J.; Fortune, B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1088–1094. [Google Scholar]
- Wang, X.; Tay, S.S.; Ng, Y.K. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Exp. Brain Res. 2000, 132, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Inman, D.M. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J. Neuroinflamm. 2018, 15, 313. [Google Scholar] [CrossRef]
- Hernandez, H.; Roberts, A.L.; McDowell, C.M. Nuclear factor-kappa beta signaling is required for transforming growth factor Beta-2 induced ocular hypertension. Exp. Eye Res. 2020, 191, 107920. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Q.; Baris, M.; Tezel, G. Transgenic inhibition of astroglial NF-κB restrains the neuroinflammatory and neurodegenerative outcomes of experimental mouse glaucoma. J. Neuroinflamm. 2020, 17, 252. [Google Scholar] [CrossRef] [PubMed]
- Mac Nair, C.E.; Schlamp, C.L.; Montgomery, A.D.; Shestopalov, V.I.; Nickells, R.W. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J. Neuroinflamm. 2016, 13, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, G.R.; Soto, I.; Zhu, X.; Ryan, M.; Macalinao, D.G.; Sousa, G.L.; Caddle, L.B.; MacNicoll, K.H.; Barbay, J.M.; Porciatti, V.; et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J. Clin. Investig. 2012, 122, 1246–1261. [Google Scholar] [CrossRef] [Green Version]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Magi, S.; Piccirillo, S.; Amoroso, S.; Lariccia, V. Excitatory amino acid transporters (EAATs): Glutamate transport and beyond. Int. J. Mol. Sci. 2019, 20, 5674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naskar, R.; Vorwerk, C.K.; Dreyer, E.B. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1940–1944. [Google Scholar]
- Harada, T.; Harada, C.; Nakamura, K.; Quah, H.M.; Okumura, A.; Namekata, K.; Saeki, T.; Aihara, M.; Yoshida, H.; Mitani, A.; et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Investig. 2007, 117, 1763–1770. [Google Scholar] [CrossRef] [Green Version]
- Osborne, N.N.; Nunez-Alvarez, C.; Joglar, B.; Del Olmo-Aguado, S. Glaucoma: Focus on mitochondria in relation to pathogenesis and neuroprotection. Eur. J. Pharmacol. 2016, 787, 127–133. [Google Scholar] [CrossRef]
- Muench, N.A.; Patel, S.; Maes, M.E.; Donahue, R.J.; Ikeda, A.; Nickells, R.W. The influence of mitochondrial dynamics and function on retinal ganglion cell susceptibility in optic nerve disease. Cells 2021, 10, 1593. [Google Scholar] [CrossRef]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Levin, L.A. Neuroprotection in glaucoma: Animal models and clinical trials. Annu. Rev. Vis. Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Noro, T.; Harada, T. Role of animal models in glaucoma research. Neural Regen. Res. 2020, 15, 1257–1258. [Google Scholar] [CrossRef]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental models of glaucoma: A powerful translational tool for the future development of new therapies for glaucoma in humans-a review of the literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, C.; Kimura, A.; Guo, X.; Namekata, K.; Harada, T. Recent advances in genetically modified animal models of glaucoma and their roles in drug repositioning. Acta Br. J. Ophthalmol. 2019, 103, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.L.; van der Merwe, Y.; Sims, J.; Parra, C.; Ho, L.C.; Schuman, J.S.; Wollstein, G.; Lathrop, K.L.; Chan, K.C. Age-related Changes in eye, brain and visuomotor behavior in the DBA/2J mouse model of chronic glaucoma. Sci. Rep. 2018, 8, 4643. [Google Scholar] [CrossRef]
- Porciatti, V.; Chou, T.H.; Feuer, W.J. C57BL/6J, DBA/2J, and DBA/2J. Gpnmb+ mice have different visual signal processing in the inner retina. Mol. Vis. 2010, 16, 2939–2947. [Google Scholar]
- Fujikawa, K.; Iwata, T.; Inoue, K.; Akahori, M.; Kadotani, H.; Fukaya, M.; Watanabe, M.; Chang, Q.; Barnett, E.M.; Swat, W. VAV2 and VAV3 as candidate disease genes for spontaneous glaucoma in mice and humans. PLoS ONE 2010, 5, e9050. [Google Scholar] [CrossRef] [Green Version]
- Reinehr, S.; Koch, D.; Weiss, M.; Froemel, F.; Voss, C.; Dick, H.B.; Fuchshofer, R.; Joachim, S.C. Loss of retinal ganglion cells in a new genetic mouse model for primary open-angle glaucoma. J. Cell. Mol. Med. 2019, 23, 5497–5507. [Google Scholar] [CrossRef] [Green Version]
- Tseng, H.C.; Riday, T.T.; McKee, C.; Braine, C.E.; Bomze, H.; Barak, I.; Marean-Reardon, C.; John, S.W.; Philpot, B.D.; Ehlers, M.D. Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol. Aging 2015, 36, 2201–2212. [Google Scholar] [CrossRef] [Green Version]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef]
- Vaghela, J.J.; Barvaliya, M.J.; Parmar, S.J.; Tripathi, C.R. Evaluation of efficacy of Aloe vera (L.) Burm. f. gel solution in methylcellulose-induced ocular hypertension in New Zealand white rabbits. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 20190158. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.C.; Marcos, H.J.; Oscar Croxatto, J.; Sande, P.H.; Campanelli, J.; Jaliffa, C.O.; Benozzi, J.; Rosenstein, R.E. A new experimental model of glaucoma in rats through intracameral injections of hyaluronic acid. Exp. Eye Res. 2005, 81, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Johnson, E.C.; Cepurna, W.O. Hypertonic saline injection model of experimental glaucoma in rats. In Glaucoma. Methods in Molecular Biology; Jakobs, T., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1695, pp. 11–21. [Google Scholar]
- Bai, Y.; Zhu, Y.; Chen, Q.; Xu, J.; Sarunic, M.V.; Saragovi, U.H.; Zhuo, Y. Validation of glaucoma-like features in the rat episcleral vein cauterization model. Chin. Med. J. 2014, 127, 359–364. [Google Scholar] [CrossRef]
- Feng, L.; Chen, H.; Suyeoka, G.; Liu, X. A laser-induced mouse model of chronic ocular hypertension to characterize visual defects. J. Vis. Exp. 2013, 10, 50440. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.; Lathrop, K.L.; Yang, E.; Sun, M.; Kagemann, L.; Fu, V.; Stolz, D.B.; Schuman, J.S.; Du, Y. A laser-induced mouse model with long-term intraocular pressure elevation. PLoS ONE 2014, 9, e107446. [Google Scholar] [CrossRef] [Green Version]
- Biermann, J.; van Oterendorp, C.; Stoykow, C.; Volz, C.; Jehle, T.; Boehringer, D.; Lagreze, W.A. Evaluation of intraocular pressure elevation in a modified laser-induced glaucoma rat model. Exp. Eye Res. 2012, 104, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Wan, K.H. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Namekata, K.; Kimura, A.; Guo, X.; Harada, C.; Murakami, A.; Matsuda, A.; Harada, T. Survival of alpha and intrinsically photosensitive retinal ganglion cells in NMDA-induced neurotoxicity and a mouse model of normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3696–3707. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Zhang, S.; Lee, C.; Kumar, A.; Arjunan, P.; Li, Y.; Zhang, F.; Li, X. An optic nerve crush injury murine model to study retinal ganglion cell survival. J. Vis. Exp. 2011, 10, e2685. [Google Scholar] [CrossRef] [Green Version]
- Rovere, G.; Nadal-Nicolas, F.M.; Agudo-Barriuso, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Nucci, C.; Villegas-Perez, M.P.; Vidal-Sanz, M. Comparison of retinal nerve fiber layer thinning and retinal ganglion cell loss after optic nerve transection in adult albino rats. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4487–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ing, E.; Ivers, K.M.; Yang, H.; Gardiner, S.K.; Reynaud, J.; Cull, G.; Wang, L.; Burgoyne, C.F. Cupping in the monkey optic nerve transection model consists of prelaminar tissue thinning in the absence of posterior laminar deformation. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2914–2927. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Guo, S.; Chai, Y.; Zhang, L.; Liu, K.; Lu, Q.; Wang, N.; Li, S. Partial optic nerve transection in rats: A model established with a new operative approach to assess secondary degeneration of retinal ganglion cells. J. Vis. Exp. 2017, 10, e56272. [Google Scholar] [CrossRef] [Green Version]
- Minhas, G.; Sharma, J.; Khan, N. Cellular stress response and immune signaling in retinal ischemia-reperfusion injury. Front. Immunol. 2016, 7, 444. [Google Scholar] [CrossRef] [Green Version]
- Hartsock, M.J.; Cho, H.; Wu, L.; Chen, W.J.; Gong, J.; Duh, E.J. A Mouse Model of Retinal Ischemia-Reperfusion Injury through Elevation of Intraocular Pressure. J. Vis. Exp. 2016, 10, e54065. [Google Scholar] [CrossRef]
- Van Bergen, N.J.; Wood, J.P.; Chidlow, G.; Trounce, I.A.; Casson, R.J.; Ju, W.K.; Weinreb, R.N.; Crowston, J.G. Recharacterization of the RGC-5 retinal ganglion cell line. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4267–4272. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.R.; Clark, A.F.; Daudt, D.; Vishwanatha, J.K.; Yorio, T. A forensic path to RGC-5 cell line identification: Lessons learned. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5712–5719. [Google Scholar] [CrossRef] [Green Version]
- Chintalapudi, S.R.; Djenderedjian, L.; Stiemke, A.B.; Steinle, J.J.; Jablonski, M.M.; Morales-Tirado, V.M. Isolation and molecular profiling of primary mouse retinal ganglion cells: Comparison of phenotypes from healthy and glaucomatous retinas. Front. Aging Neurosci. 2016, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef] [PubMed]
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition—Chapter 3: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. Acta Br. J. Ophthalmol. 2017, 101, 130–195. [CrossRef] [PubMed] [Green Version]
- Wan, M.J.; Daniel, S.; Kassam, F.; Mutti, G.; Butty, Z.; Kasner, O.; Trope, G.E.; Buys, Y.M. Survey of complementary and alternative medicine use in glaucoma patients. J. Glaucoma 2012, 21, 79–82. [Google Scholar] [CrossRef]
- AlSalman, S.; AlHussaini, M.A.; Khandekar, R.B.; Edward, D.P. The proportion of complementary and alternative medicine utilization among Saudi population for eye care: Cross-sectional study. Cureus 2021, 13, e13109. [Google Scholar] [CrossRef] [PubMed]
- Jaber, D.; Ghannam, R.A.; Rashed, W.; Shehadeh, M.; Zyoud, S.H. Use of complementary and alternative therapies by patients with eye diseases: A hospital-based cross-sectional study from Palestine. BMC Complementary Med. Ther. 2021, 21, 3. [Google Scholar] [CrossRef]
- Achete de Souza, G.; de Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo biloba on diseases related to oxidative stress. Planta Med. 2020, 86, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.G.; Wu, S.Q.; Li, P.; Yang, H. Advancement in the chemical analysis and quality control of flavonoid in Ginkgo biloba. J. Pharm. Biomed. Anal. 2015, 113, 212–225. [Google Scholar] [CrossRef]
- Cho, H.K.; Kim, S.; Lee, E.J.; Kee, C. Neuroprotective effect of Ginkgo biloba extract against hypoxic retinal ganglion cell degeneration in vitro and in vivo. J. Med. Food 2019, 22, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Neutzner, A.; Turtschi, S.; Flammer, J.; Mozaffarieh, M. The effect of Ginkgo biloba and Nifedipine on DNA breaks in circulating leukocytes of glaucoma patients. Expert Rev. Ophthalmol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, S.W.; Kee, C. Effect of Ginkgo biloba extract on visual field progression in normal tension glaucoma. J. Glaucoma 2013, 22, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food 2012, 15, 818–823. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Kwon, H.J.; Chung, W.S.; Kim, C.Y.; Seong, G.J. Short-term effects of Ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J. Ophthalmol. 2011, 25, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaner, M.C.; Dogan, M.; Altin, S.S.; Balaman, C.; Yilmaz, C.; Omur, A.; Zeybek, I.; Palaz, M. Ginkgo Biloba affects microvascular morphology: A prospective optical coherence tomography angiography pilot study. Int. Ophthalmol. 2021, 41, 1053–1061. [Google Scholar] [CrossRef]
- Ma, K.; Xu, L.; Zhang, H.; Zhang, S.; Pu, M.; Jonas, J.B. The effect of ginkgo biloba on the rat retinal ganglion cell survival in the optic nerve crush model. Acta Ophthalmol. 2010, 88, 553–557. [Google Scholar] [CrossRef]
- Fan, X.X.; Cao, Z.Y.; Liu, M.X.; Liu, W.J.; Xu, Z.L.; Tu, P.F.; Wang, Z.Z.; Cao, L.; Xiao, W. Diterpene Ginkgolides Meglumine Injection inhibits apoptosis induced by optic nerve crush injury via modulating MAPKs signaling pathways in retinal ganglion cells. J. Ethnopharmacol. 2021, 279, 114371. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Z.; Wang, K.; Zhu, X.; Ali, Y.; Shu, W.; Bao, X.; Zhu, L.; Fan, X.; Murray, M.; et al. Procyanidin B2 and rutin in Ginkgo biloba extracts protect human retinal pigment epithelial (RPE) cells from oxidative stress by modulating Nrf2 and Erk1/2 signalling. Exp. Eye Res. 2021, 207, 108586. [Google Scholar] [CrossRef]
- Yu, H.; Dong, H.; Zhang, Y.; Liu, Q. A network pharmacology-based strategy for predicting the protective mechanism of Ginkgo biloba on damaged retinal ganglion cells. Chin. J. Nat. Med. 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Xiao, J.R.; Do, C.W.; To, C.H. Potential therapeutic effects of baicalein, baicalin, and wogonin in ocular disorders. J. Ocul. Pharmacol. Ther. 2014, 30, 605–614. [Google Scholar] [CrossRef]
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, baicalin, and wogonin: Protective effects against ischemia-induced neurodegeneration in the brain and retina. Oxidative Med. Cell. Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.S.; Lee, D.; Kim, H. Safety evaluation of root extract of Pueraria lobata and Scutellaria baicalensis in rats. BMC Complementary Med. Ther. Vol. 2020, 20, 226. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, M.; Song, Q.; Li, S.; Zhao, X.; Kan, L.; Zhu, S. Integrating network pharmacological and experimental models to investigate the therapeutic effects of baicalein in glaucoma. Chin. Med. 2021, 16, 124. [Google Scholar] [CrossRef]
- Chao, H.M.; Chuang, M.J.; Liu, J.H.; Liu, X.Q.; Ho, L.K.; Pan, W.H.; Zhang, X.M.; Liu, C.M.; Tsai, S.K.; Kong, C.W.; et al. Baicalein protects against retinal ischemia by antioxidation, antiapoptosis, downregulation of HIF-1alpha, VEGF, and MMP-9 and upregulation of HO-1. J. Ocul. Pharmacol. Ther. 2013, 29, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yang, B.; Hu, Y.; Lu, L.; Lu, X.; Wang, J.; Xu, F.; Yu, S.; Huang, J.; Liang, X. Wogonin prevents TLR4-NF-κB-medicated neuro-inflammation and improves retinal ganglion cells survival in retina after optic nerve crush. Oncotarget 2016, 7, 72503–72517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, N.; Shi, J.; Xu, H.; Luo, Q.; Li, Q.; Liu, M. Baicalin suppresses glaucoma pathogenesis by regulating the PI3K/AKT signaling in vitro and in vivo. Bioengineered 2021, 12, 10187–10198. [Google Scholar] [CrossRef]
- Gong, L.; Zhu, J. Baicalin alleviates oxidative stress damage in trabecular meshwork cells in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 51–58. [Google Scholar] [CrossRef]
- Srivastava, S.; Misra, A.; Mishra, P.; Shukla, P.; Kumar, M.; Sundaresan, V.; Negi, K.S.; Agrawal, P.K.; Rawat, A.K.S. Molecular and chemotypic variability of forskolin in Coleus forskohlii Briq., a high value industrial crop collected from Western Himalayas (India). RSC Adv. 2017, 7, 8843–8851. [Google Scholar] [CrossRef] [Green Version]
- Shim, M.S.; Kim, K.Y.; Ju, W.K. Role of cyclic AMP in the eye with glaucoma. BMB Rep. 2017, 50, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidullah, M.; Wilson, W.S.; Rafiq, K.; Sikder, M.H.; Ferdous, J.; Delamere, N.A. Terbutaline, forskolin and cAMP reduce secretion of aqueous humour in the isolated bovine eye. PLoS ONE 2020, 15, e0244253. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Kumar, S.K. A double-blind, randomized clinical trial to evaluate the efficacy and safety of forskolin eye drops 1% in the treatment of open angle glaucoma—A comparative study. J. Clin. Trials 2014, 4, 1000184. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Karri, S.K.; Jose, J.A. Efficacy and safety of 1% forskolin eye drops in open angle glaucoma—An open label study. Saudi J. Ophthalmol. 2015, 29, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Locri, F.; Cammalleri, M.; Dal Monte, M.; Rusciano, D.; Bagnoli, P. Protective efficacy of a dietary supplement based on forskolin, homotaurine, spearmint extract, and group B vitamins in a mouse model of optic nerve injury. Nutrients 2019, 11, 2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammalleri, M.; Dal Monte, M.; Amato, R.; Bagnoli, P.; Rusciano, D. A dietary combination of forskolin with homotaurine, spearmint and B vitamins protects injured retinal ganglion cells in a rodent model of hypertensive glaucoma. Nutrients 2020, 12, 1189. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Lin, P.; Kang, Q.; Zhao, Z.L.; Wang, J.; Cheng, J.Y. Metabolism and pharmacological mechanisms of active ingredients in Erigeron breviscapus. Curr. Drug Metab. 2021, 22, 24–39. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiang, M.; Ye, W.; Cheng, Y.; Jiang, Y. Visual field protective effect of Erigeron breviscapus (vant.) Hand. Mazz. extract on glaucoma with controlled intraocular pressure: A randomized, double-blind, clinical trial. Drugs R D 2010, 10, 75–82. [Google Scholar] [CrossRef]
- Lu, X.J.; Zhang, F.W.; Cheng, L.; Liu, A.Q.; Duan, J.G. Effect on multifocal electroretinogram in persistently elevated intraocular pressure by erigeron breviscapus extract. J. Ophthalmol. 2011, 4, 349–352. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiang, M.; Ye, W.; Huang, P.; Cheng, Y.; Jiang, Y. Neuroprotective effect of Erigeron Breviscapus (vant) Hand-mazz extract on retinal ganglion cells in rabbits with chronic elevated intraocular pressure. Asian Biomed. 2011, 5, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Wang, Z.F.; Duan, J.G.; Ji, L.; Lu, X.J. Extraction (DSX) from Erigeron breviscapus modulates outward potassium currents in rat retinal ganglion cells. Int. J. Ophthalmol. 2015, 8, 1101–1106. [Google Scholar] [CrossRef]
- Zhu, J.; Sainulabdeen, A.; Akers, K.; Adi, V.; Sims, J.R.; Yarsky, E.; Yan, Y.; Yu, Y.; Ishikawa, H.; Leung, C.K.; et al. Oral scutellarin treatment ameliorates retinal thinning and visual deficits in experimental glaucoma. Front. Med. 2021, 8, 681169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, L.; Qi, Y.; Feng, J.; Zhu, L.; Bai, Y.; Wu, H. Protective effects of Erigeron breviscapus Hand.- Mazz. (EBHM) extract in retinal neurodegeneration models. Mol. Vis. 2018, 24, 315–325. [Google Scholar]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [Green Version]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crisan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.S.; Feng, Q.; Lo, A.C.; Chang, R.C.; Lin, B.; Chung, S.K.; So, K.F. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS ONE 2012, 7, e45469. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, Y.; Wong, F.S.; Yu, W.Y.; Li, S.Z.; Choi, K.Y.; So, K.F.; Chan, H.H. Lycium barbarum polysaccharides rescue neurodegeneration in an acute ocular hypertension rat model under pre- and posttreatment conditions. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.S.; Chiu, K.; Van, G.; Leung, J.W.; Lo, A.C.; Chung, S.K.; Chang, R.C.; So, K.F. Effect of Lycium barbarum polysaccharides on the expression of endothelin-1 and its receptors in an ocular hypertension model of rat glaucoma. Neural Regen. Res. 2012, 7, 645–651. [Google Scholar] [CrossRef]
- Lakshmanan, Y.; Wong, F.S.Y.; Zuo, B.; So, K.F.; Bui, B.V.; Chan, H.H. Posttreatment intervention with Lycium barbarum polysaccharides is neuroprotective in a rat model of chronic ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4606–4618. [Google Scholar] [CrossRef]
- Chu, P.H.; Li, H.Y.; Chin, M.P.; So, K.F.; Chan, H.H. Effect of Lycium barbarum (wolfberry) polysaccharides on preserving retinal function after partial optic nerve transection. PLoS ONE 2013, 8, e81339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liang, Y.; Chiu, K.; Yuan, Q.; Lin, B.; Chang, R.C.; So, K.F. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS ONE 2013, 8, e68881. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Sha, X.Y.; Wu, Y.N.; Chen, M.T.; Zhong, J.X. Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury. Neural Regen. Res. 2020, 15, 1526–1531. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y. Lycium barbarum polysaccharides alleviate hydrogen peroxide-induced injury by up-regulation of miR-4295 in human trabecular meshwork cells. Exp. Mol. Pathol. 2019, 106, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Huang, M.; Luo, Q.Y.; Hong, X.; Ramakrishna, S.; So, K.F. Lycium barbarum (Wolfberry) increases retinal ganglion cell survival and affects both microglia/macrophage polarization and autophagy after rat partial optic nerve transection. Cell Transpl. 2019, 28, 607–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, X.S.; Feng, Q.; Lo, A.C.Y.; Chang, R.C.; Chung, S.K.; So, K.F. Lycium barbarum polysaccharides related RAGE and Abeta levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier. Neural Regen. Res. 2020, 15, 2344–2352. [Google Scholar] [CrossRef]
- Xu, S.; Liu, S.; Yan, G. Lycium barbarum exerts protection against glaucoma-like injury via inhibition of MMP-9 signaling in vitro. Med. Sci. Monit. 2019, 25, 9794–9800. [Google Scholar] [CrossRef] [PubMed]
- Comes, N.; Buie, L.K.; Borras, T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: Implications for glaucoma. Genes Cells 2011, 16, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R.N.; Robinson, M.R.; Dibas, M.; Stamer, W.D. Matrix metalloproteinases and glaucoma treatment. J. Ocul. Pharmacol. Ther. 2020, 36, 208–228. [Google Scholar] [CrossRef]
- Yang, D.; So, K.F.; Lo, A.C. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin. Exp. Ophthalmol. 2017, 45, 717–729. [Google Scholar] [CrossRef]
- He, M.; Pan, H.; Chang, R.C.; So, K.F.; Brecha, N.C.; Pu, M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS ONE 2014, 9, e84800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, I.H.; Chan, S.M.; Lin, C.T. The neuroprotective effect of submicron and blended Lycium barbarum for experiment retinal ischemia and reperfusion injury in rats. J. Vet. Med. Sci. 2020, 82, 1719–1728. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; Andrade, C.J.d.; Miyahira, R.F.; Fai, A.E.C. Persimmon (Diospyros Kaki L.): Chemical Properties, Bioactive Compounds and Potential Use in the Development of New Products—A Review. Food Rev. Int. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Hossain, A.; Moon, H.K.; Kim, J.K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184. [Google Scholar] [CrossRef]
- Ryul Ahn, H.; Kim, K.A.; Kang, S.W.; Lee, J.Y.; Kim, T.J.; Jung, S.H. Persimmon leaves (Diospyros kaki) extract protects optic nerve crush-induced retinal degeneration. Sci. Rep. 2017, 7, 46449. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.R.; Yang, J.W.; Kim, J.Y.; Lee, C.Y.; Kim, T.J.; Jung, S.H. The intraocular pressure-lowering effect of persimmon leaves (Diospyros kaki) in a mouse model of glaucoma. Int. J. Mol. Sci. 2019, 20, 5268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hao, J. Treatment of neurodegenerative diseases with bioactive components of Tripterygium wilfordii. Am. J. Chin. Med. 2019, 47, 769–785. [Google Scholar] [CrossRef]
- Chen, S.R.; Dai, Y.; Zhao, J.; Lin, L.; Wang, Y.; Wang, Y. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front. Pharmacol. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Wu, L.; Guo, X.; Wang, D.; Li, Y. Improved retinal ganglion cell survival through retinal microglia suppression by a chinese herb extract, triptolide, in the DBA/2J mouse model of glaucoma. Ocul. Immunol. Inflamm. 2013, 21, 378–389. [Google Scholar] [CrossRef]
- Yang, F.; Wang, D.; Wu, L.; Li, Y. Protective effects of triptolide on retinal ganglion cells in a rat model of chronic glaucoma. Drug Des. Dev. Ther. 2015, 9, 6095–6107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.F.; Zou, Y.F.; Chen, X.F.; Zhang, W. Effect of triptolide on retinal ganglion cell survival in an optic nerve crush model. Cell Mol. Biol. 2017, 63, 102–107. [Google Scholar] [CrossRef]
- Kyung, H.; Kwong, J.M.; Bekerman, V.; Gu, L.; Yadegari, D.; Caprioli, J.; Piri, N. Celastrol supports survival of retinal ganglion cells injured by optic nerve crush. Brain Res. 2015, 1609, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Kwong, J.M.K.; Yadegari, D.; Yu, F.; Caprioli, J.; Piri, N. The effect of celastrol on the ocular hypertension-induced degeneration of retinal ganglion cells. Neurosci. Lett. 2018, 670, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Rasool, A.; Imran Mir, M.; Zulfajri, M.; Hanafiah, M.M.; Azeem Unnisa, S.; Mahboob, M. Plant growth promoting and antifungal asset of indigenous rhizobacteria secluded from saffron (Crocus sativus L.) rhizosphere. Microb. Pathog. 2021, 150, 104734. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Albarral, J.A.; Ramirez, A.I.; de Hoz, R.; Lopez-Villarin, N.; Salobrar-Garcia, E.; Lopez-Cuenca, I.; Licastro, E.; Inarejos-Garcia, A.M.; Almodovar, P.; Pinazo-Duran, M.D.; et al. Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef] [Green Version]
- Jabbarpoor Bonyadi, M.H.; Yazdani, S.; Saadat, S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: A pilot study. BMC Complement. Altern. Med. 2014, 14, 399. [Google Scholar] [CrossRef] [Green Version]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical composition and neuroprotective activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Y.; Yang, X. Neuroprotective effects of crocin against oxidative stress induced by ischemia/reperfusion injury in rat retina. Ophthalmic Res. 2015, 54, 157–168. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, L.; Zhang, L.; Liu, W.B.; Chen, X.Y.; Yang, X.G. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp. Eye Res. 2013, 107, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Chen, T.; Xu, Z.; Huo, F.; Wei, Y.; Yang, X. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int. J. Mol. Med. 2016, 37, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Nakanishi, T.; Umigai, N.; Tsuruma, K.; Shimazawa, M.; Hara, H. Oral administration of crocetin prevents inner retinal damage induced by N-methyl-D-aspartate in mice. Eur. J. Pharmacol. 2012, 690, 84–89. [Google Scholar] [CrossRef]
- Ishizuka, F.; Shimazawa, M.; Umigai, N.; Ogishima, H.; Nakamura, S.; Tsuruma, K.; Hara, H. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur. J. Pharmacol. 2013, 703, 1–10. [Google Scholar] [CrossRef]
- Kevin, T.T.M.; Nur Idanis, A.S.; Anastasha, B.; Mohd Faris, M.R.; Faizah, O.; Taty Anna, K. Curcumin minimises histopathological and immunological progression in the ankle joints of collagen-induced arthritis rats. Med. Health 2020, 15, 26–36. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.; Mokhtar, M.H. Potential health benefits of curcumin on female reproductive disorders: A review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef]
- Yue, Y.K.; Mo, B.; Zhao, J.; Yu, Y.J.; Liu, L.; Yue, C.L.; Liu, W. Neuroprotective effect of curcumin against oxidative damage in BV-2 microglia and high intraocular pressure animal model. J. Ocul. Pharmacol. Ther. 2014, 30, 657–664. [Google Scholar] [CrossRef]
- Buccarello, L.; Dragotto, J.; Hassanzadeh, K.; Maccarone, R.; Corbo, M.; Feligioni, M. Retinal ganglion cell loss in an ex vivo mouse model of optic nerve cut is prevented by curcumin treatment. Cell Death Discov. 2021, 7, 394. [Google Scholar] [CrossRef]
- Esfandiari, A.; Hashemi, F. Protective effects of curcumin on ischemic reperfusion of rat retina. Comp. Clin. Pathol. 2019, 28, 89–95. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Guo, H.; Kern, T.S.; Huang, K.; Zheng, L. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS ONE 2011, 6, e23194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Wu, X. Curcumin protects trabecular meshwork cells from oxidative stress. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4327–4332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Ding, H.; Li, D.; Luo, J. Curcumin protects trabecular meshwork cells against hydrogen peroxide-induced oxidative stress and apoptosis via Nrf2-keap1 pathway. Trop. J. Pharm. Res. 2018, 17, 2169–2176. [Google Scholar] [CrossRef]
- Azmi, N.; Chee, S.H.; Mohd Fauzi, N.; Jasamai, M.; Kumolosasi, E. Viability and apoptotic effects of green tea (Camellia sinensis) methanol extract on human leukemic cell lines. Acta Pol. Pharm. Drug Res. 2018, 75, 51–58. [Google Scholar]
- Yang, Y.; Xu, C.; Chen, Y.; Liang, J.J.; Xu, Y.; Chen, S.L.; Huang, S.; Yang, Q.; Cen, L.P.; Pang, C.P.; et al. Green tea extract ameliorates ischemia-induced retinal ganglion cell degeneration in rats. Oxidative Med. Cell. Longev. 2019, 2019, 8407206. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.L.; Yu, Q.X.; Liang, W.C.; Leung, P.Y.; Ng, T.K.; Chu, W.K.; Pang, C.P.; Chan, S.O. Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci. Rep. 2018, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Omar, M.S.; Adnan, N.N.; Kumolosasi, E.; Azmi, N.; Damanhuri, N.S.; Buang, F. Green tea (Camellia sinensis) extract reduces peptic ulcer induced by Helicobacter pylori in Sprague Dawley rats. Sains Malays. 2020, 49, 2793–2800. [Google Scholar] [CrossRef]
- Zhang, W.H.; Chen, Y.; Gao, L.M.; Cao, Y.N. Neuroprotective role of epigallocatechin-3-gallate in acute glaucoma via the nuclear factor-κB signalling pathway. Exp. Ther. Med. 2021, 22, 1235. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, L.; Jiang, L.; Lai, T.Y. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci. Lett. 2015, 600, 132–136. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, L.; Zhang, T.; Jin, Y.; Yang, D.; Chen, F. Neuroprotective effects of Epigallocatechin-3-gallate (EGCG) in optic nerve crush model in rats. Neurosci. Lett. 2010, 479, 26–30. [Google Scholar] [CrossRef]
- Rivera-Perez, J.; Martinez-Rosas, M.; Conde-Castanon, C.A.; Toscano-Garibay, J.D.; Ruiz-Perez, N.J.; Flores, P.L.; Mera Jimenez, E.; Flores-Estrada, J. Epigallocatechin 3-Gallate has a neuroprotective effect in retinas of rabbits with ischemia/reperfusion through the activation of Nrf2/HO-1. Int. J. Mol. Sci. 2020, 21, 3716. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, L.; Shen, C.; Wan, H.; Xu, L.; Wang, N.; Jonas, J.B. Neuroprotective effect of epigallocatechin-3-gallate against N-methyl-D-aspartate-induced excitotoxicity in the adult rat retina. Acta Ophthalmol. 2012, 90, e609–e615. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed. Pharmacother. 2017, 85, 120–127. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kim, D.Y.; Lee, J.S.; Hwang, S.J.; Kim, G.H.; Hyun, S.H.; Son, C.G. Korean red ginseng ameliorates fatigue via modulation of 5-HT and corticosterone in a sleep-deprived mouse model. Nutrients 2021, 13, 3121. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yang, H.; Kim, J.Y.; Choi, W.; Seong, G.J.; Kim, C.Y.; Lee, J.M.; Bae, H.W. Effect of red ginseng on visual function and vision-related quality of life in patients with glaucoma. J. Ginseng Res. 2021, 45, 676–682. [Google Scholar] [CrossRef]
- Bae, H.W.; Kim, J.H.; Kim, S.; Kim, M.; Lee, N.; Hong, S.; Seong, G.J.; Kim, C.Y. Effect of Korean red ginseng supplementation on dry eye syndrome in glaucoma patients—A randomized, double-blind, placebo-controlled study. J. Ginseng Res. 2015, 39, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.R.; Kim, J.H.; Kim, C.Y. Effect of Korean red ginseng supplementation on ocular blood flow in patients with glaucoma. J. Ginseng Res. 2010, 34, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Mathiyalagan, R.; Yang, D.C. Ginseng nanoparticles: A budding tool for cancer treatment. Nanomedicine 2017, 12, 1091–1094. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Yu, H.; Chen, B.; Guo, L.; Xu, X.; Jiang, M.; Zhong, Y.; Qi, J.; Huang, P. Protective effect of total Panax notoginseng saponins on retinal ganglion cells of an optic nerve crush injury rat model. Biomed Res. Int. 2021, 2021, 4356949. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Chen, H. Treatment of glaucomatous optic nerve damage using ginsenoside Rg1 mediated by ultrasound targeted microbubble destruction. Exp. Ther. Med. 2018, 15, 300–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Chen, J.; Huang, W.; Zeng, Z.; Yang, Y.; Zhu, B. Ginsenoside Rb1 protects rat retinal ganglion cells against hypoxia and oxidative stress. Mol. Med. Rep. 2013, 8, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill-Dee, C.; Spiller, H.A.; Casavant, M.J.; Kistamgari, S.; Chounthirath, T.; Smith, G.A. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000–2017. Clin. Toxicol. 2019, 58, 813–820. [Google Scholar] [CrossRef]
- Katz, J.; Costarides, A.P. Facts vs fiction: The role of cannabinoids in the treatment of glaucoma. Curr. Ophthalmol. Rep. 2019, 7, 177–181. [Google Scholar] [CrossRef]
- Merritt, J.C.; Crawford, W.J.; Alexander, P.C.; Anduze, A.L.; Gelbart, S.S. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology 1980, 87, 222–228. [Google Scholar] [CrossRef]
- Mosaed, S.; Liu, J.H.K.; Minckler, D.S.; Fitzgerald, R.L.; Grelotti, D.; Sones, E.; Sheils, C.R.; Weinreb, R.N.; Marcotte, T.D. The effect of inhaled cannabis on intraocular pressure in healthy adult subjects. Ophthalmology 2021, 15, 33–37. [Google Scholar] [CrossRef]
- Fischer, K.M.; Ward, D.A.; Hendrix, D.V. Effects of a topically applied 2% delta-9-tetrahydrocannabinol ophthalmic solution on intraocular pressure and aqueous humor flow rate in clinically normal dogs. Am. J. Vet. Res. 2013, 74, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.; Dudhipala, N.; Thakkar, R.; Mehraj, T.; Marathe, S.; Gul, W.; ElSohly, M.A.; Murphy, B.; Majumdar, S. Effect of surfactant concentration and sterilization process on intraocular pressure-lowering activity of Delta(9)-tetrahydrocannabinol-valine-hemisuccinate (NB1111) nanoemulsions. Drug Deliv. Transl. Res. 2021, 11, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, S.; Almog, S.; Torracca, M.T.; Saettone, M.F.; Benita, S. A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: Effect on intraocular pressure in rabbits. Ophthalmic Res. 1992, 24, 142–149. [Google Scholar] [CrossRef]
- Song, Z.H.; Slowey, C.A. Involvement of cannabinoid receptors in the intraocular pressure-lowering effects of WIN55212-2. J. Pharmacol. Exp. Ther. 2000, 292, 136–139. [Google Scholar]
- Pinar-Sueiro, S.; Zorrilla Hurtado, J.A.; Veiga-Crespo, P.; Sharma, S.C.; Vecino, E. Neuroprotective effects of topical CB1 agonist WIN 55212-2 on retinal ganglion cells after acute rise in intraocular pressure induced ischemia in rat. Exp. Eye Res. 2013, 110, 55–58. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Eng Khoo, H.; Meng Lim, S.; Azlan, A. Evidence-Based Therapeutic Effects of Anthocyanins from Foods. Pak. J. Nutr. 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Ohguro, I.; Yagi, S. Effects of black currant anthocyanins on intraocular pressure in healthy volunteers and patients with glaucoma. J. Ocul. Pharmacol. Ther. 2013, 29, 61–67. [Google Scholar] [CrossRef]
- Yoshida, K.; Ohguro, I.; Ohguro, H. Black currant anthocyanins normalized abnormal levels of serum concentrations of endothelin-1 in patients with glaucoma. J. Ocul. Pharmacol. Ther. 2013, 29, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.H.; Wu, A.L.; Wang, N.K.; Chen, K.J.; Liu, L.; Hwang, Y.S.; Yeung, L.; Wu, W.C.; Lai, C.C. The intraocular staining potential of anthocyanins and their retinal biocompatibility: A preclinical study. Cutan. Ocul. Toxicol. 2018, 37, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, O.; Moritoh, S.; Sato, K.; Maekawa, S.; Murayama, N.; Himori, N.; Omodaka, K.; Sogon, T.; Nakazawa, T. Bilberry extract administration prevents retinal ganglion cell death in mice via the regulation of chaperone molecules under conditions of endoplasmic reticulum stress. Clin. Ophthalmol. 2017, 11, 1825–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, A.; Santhanathas, T.; Shaukat Ali, S.; Zainalabidin, S. Resveratrol supplementation protects against nicotine-induced kidney injury. Int. J. Environ. Res Public Health 2019, 16, 4445. [Google Scholar] [CrossRef] [Green Version]
- Avotri, S.; Eatman, D.; Russell-Randall, K. Effects of Resveratrol on inflammatory biomarkers in glaucomatous human trabecular meshwork cells. Nutrients 2019, 11, 984. [Google Scholar] [CrossRef] [Green Version]
- Pirhan, D.; Yuksel, N.; Emre, E.; Cengiz, A.; Kursat Yildiz, D. Riluzole- and resveratrol-induced delay of retinal ganglion cell death in an experimental model of glaucoma. Curr. Eye Res. 2016, 41, 59–69. [Google Scholar] [CrossRef]
- Cao, K.; Ishida, T.; Fang, Y.; Shinohara, K.; Li, X.; Nagaoka, N.; Ohno-Matsui, K.; Yoshida, T. Protection of the retinal ganglion cells: Intravitreal injection of resveratrol in mouse model of ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.J.; Meng, N. Resveratrol acts via the mitogen-activated protein kinase (MAPK) pathway to protect retinal ganglion cells from apoptosis induced by hydrogen peroxide. Bioengineered 2021, 12, 4878–4886. [Google Scholar] [CrossRef]
- Ji, K.; Li, Z.; Lei, Y.; Xu, W.; Ouyang, L.; He, T.; Xing, Y. Resveratrol attenuates retinal ganglion cell loss in a mouse model of retinal ischemia reperfusion injury via multiple pathways. Exp. Eye Res. 2021, 209, 108683. [Google Scholar] [CrossRef]
- Luo, H.; Zhuang, J.; Hu, P.; Ye, W.; Chen, S.; Pang, Y.; Li, N.; Deng, C.; Zhang, X. Resveratrol delays retinal ganglion cell loss and attenuates gliosis-related inflammation from ischemia-reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3879–3888. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Yang, X.; Chen, W. Resveratrol protects the retina from I/R injury by inhibiting RGCS apoptosis, glial activation and expression of inflammatory factors. Trop. J. Pharm. Res. 2020, 19, 1221–1226. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Wang, Y.; Wang, J.; Xiang, D.; Niu, W.; Yuan, F. Resveratrol ameliorates disorders of mitochondrial biogenesis and dynamics in a rat chronic ocular hypertension model. Life Sci. 2018, 207, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Qin, M.; Hu, P.; Ji, K.; Xiao, R.; Sun, N.; Pan, X.; Zhang, X. Resveratrol protects retinal ganglion cells against ischemia induced damage by increasing Opa1 expression. Int. J. Mol. Med. 2020, 46, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Means, J.C.; Lopez, A.A.; Koulen, P. Resveratrol protects optic nerve head astrocytes from oxidative stress-induced cell death by preventing caspase-3 activation, tau dephosphorylation at Ser(422) and formation of misfolded protein aggregates. Cell. Mol. Neurobiol. 2020, 40, 911–926. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Himori, N.; Inoue Yanagimachi, M.; Omodaka, K.; Shiga, Y.; Tsuda, S.; Kunikata, H.; Nakazawa, T. The effect of dietary antioxidant supplementation in patients with glaucoma. Clin. Ophthalmol. 2021, 15, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, X.; Ren, Z.; Jiang, H.; Liu, B. Anti-glaucoma potential of hesperidin in experimental glaucoma induced rats. AMB Express 2020, 10, 94. [Google Scholar] [CrossRef]
- Sato, K.; Sato, T.; Ohno-Oishi, M.; Ozawa, M.; Maekawa, S.; Shiga, Y.; Yabana, T.; Yasuda, M.; Himori, N.; Omodaka, K.; et al. CHOP deletion and anti-neuroinflammation treatment with hesperidin synergistically attenuate NMDA retinal injury in mice. Exp. Eye Res. 2021, 213, 108826. [Google Scholar] [CrossRef]
- Maekawa, S.; Sato, K.; Fujita, K.; Daigaku, R.; Tawarayama, H.; Murayama, N.; Moritoh, S.; Yabana, T.; Shiga, Y.; Omodaka, K.; et al. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci. Rep. 2017, 7, 6885. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Li, Y.; Liu, H. Hesperidin ameliorates hypobaric hypoxia-induced retinal impairment through activation of Nrf2/HO-1 pathway and inhibition of apoptosis. Sci. Rep. 2020, 10, 19426. [Google Scholar] [CrossRef] [PubMed]
- Md Isa, Z.; Anuar, A.A.; Danial Azmi, A.; Selvan, S.T.; Hisham, N.S.; Yong, Z.Q. Does caffeine intake influence mental health of medical students? Malays. J. Public Health Med. 2021, 21, 22–28. [Google Scholar] [CrossRef]
- Tran, T.; Niyadurupola, N.; O’Connor, J.; Ang, G.S.; Crowston, J.; Nguyen, D. Rise of intraocular pressure in a caffeine test versus the water drinking test in patients with glaucoma. Clin. Exp. Ophthalmol. 2014, 42, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Gaur, A.; Varma, S. Effect of caffeine on the intraocular pressure in patients with primary open angle glaucoma. Clin. Ophthalmol. 2011, 5, 1623–1629. [Google Scholar] [CrossRef] [Green Version]
- Vera, J.; Redondo, B.; Molina, R.; Bermudez, J.; Jimenez, R. Effects of caffeine on intraocular pressure are subject to tolerance: A comparative study between low and high caffeine consumers. Psychopharmacology 2019, 236, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Redondo, B.; Vera, J.; Molina, R.; Jimenez, R. Short-term effects of caffeine intake on anterior chamber angle and intraocular pressure in low caffeine consumers. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Aschard, H.; Kang, J.H.; Lentjes, M.A.H.; Do, R.; Wiggs, J.L.; Khawaja, A.P.; Pasquale, L.R.; Modifiable Risk Factors for Glaucoma Collaboration. Intraocular pressure, glaucoma, and dietary caffeine consumption: A gene-diet interaction study from the UK Biobank. Ophthalmology 2021, 128, 866–876. [Google Scholar] [CrossRef]
- Nakano, E.; Miyake, M.; Hosoda, Y.; Mori, Y.; Suda, K.; Kameda, T.; Ikeda-Ohashi, H.; Tabara, Y.; Yamashiro, K.; Tamura, H.; et al. Relationship between intraocular pressure and coffee consumption in a Japanese population without glaucoma: The Nagahama study. Ophthalmol. Glaucoma 2021, 4, 268–276. [Google Scholar] [CrossRef]
- Madeira, M.H.; Ortin-Martinez, A.; Nadal-Nicolas, F.; Ambrosio, A.F.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Santiago, A.R. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci. Rep. 2016, 6, 27532. [Google Scholar] [CrossRef]
- Boia, R.; Elvas, F.; Madeira, M.H.; Aires, I.D.; Rodrigues-Neves, A.C.; Tralhao, P.; Szabo, E.C.; Baqi, Y.; Muller, C.E.; Tome, A.R.; et al. Treatment with A2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell Death Dis. 2017, 8, e3065. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.; Lazzara, F.; Romano, G.L.; Platania, C.B.M.; Drago, F.; Bucolo, C. Caffeine protects against retinal inflammation. Front. Pharmacol. 2022, 12, 824885. [Google Scholar] [CrossRef]
- Zulfakar, M.H.; Chan, L.M.; Rehman, K.; Wai, L.K.; Heard, C.M. Coenzyme Q10-loaded fish oil-based bigel system: Probing the delivery across porcine skin and possible interaction with fish oil fatty acids. AAPS PharmSciTech. 2018, 19, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Ekeuku, S.O.; Ima-Nirwana, S.; Chin, K.Y. Skeletal protective effect of Coenzyme Q10: A review. Int. J. Pharmacol. 2020, 16, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Kim, K.Y.; Shim, M.S.; Kim, S.Y.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 ameliorates oxidative stress and prevents mitochondrial alteration in ischemic retinal injury. Apoptosis 2014, 19, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, B.M.; Tian, K.; Pahlitzsch, M.; Brenton, J.; Ravindran, N.; Butt, G.; Malaguarnera, G.; Normando, E.M.; Guo, L.; Cordeiro, M.F. Topical Coenzyme Q10 demonstrates mitochondrial-mediated neuroprotection in a rodent model of ocular hypertension. Mitochondrion 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Parisi, V.; Centofanti, M.; Gandolfi, S.; Marangoni, D.; Rossetti, L.; Tanga, L.; Tardini, M.; Traina, S.; Ungaro, N.; Vetrugno, M.; et al. Effects of coenzyme Q10 in conjunction with vitamin E on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J. Glaucoma 2014, 23, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Ekicier Acar, S.; Saricaoglu, M.S.; Colak, A.; Aktas, Z.; Sepici Dincel, A. Neuroprotective effects of topical coenzyme Q10 + vitamin E in mechanic optic nerve injury model. Eur. J. Ophthalmol. 2020, 30, 714–722. [Google Scholar] [CrossRef]
- Wang, S.Y.; Singh, K.; Lin, S.C. Glaucoma and vitamins A, C, and E supplement intake and serum levels in a population-based sample of the United States. Eye 2013, 27, 487–494. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Shao, M.; Cao, W.; Sun, X. Lack of association between serum vitamin B6, vitamin B12, and vitamin D levels with different types of glaucoma: A systematic review and meta-analysis. Nutrients 2017, 9, 636. [Google Scholar] [CrossRef] [Green Version]
- Ramdas, W.D.; Schouten, J.; Webers, C.A.B. The effect of vitamins on glaucoma: A systematic review and meta-analysis. Nutrients 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.H.; Romano, G.L.; Amato, R.; Porciatti, V. Nicotinamide-rich diet in DBA/2J mice preserves retinal ganglion cell metabolic function as assessed by PERG adaptation to flicker. Nutrients 2020, 12, 1910. [Google Scholar] [CrossRef]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- Vukovic Arar, Z.; Knezevic Pravecek, M.; Miskic, B.; Vatavuk, Z.; Vukovic Rodriguez, J.; Sekelj, S. Association between serum vitamin D level and glaucoma in women. Acta Clin. Croat. 2016, 55, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Yao, Q.; Ma, W.; Liu, H.; Ji, J.; Li, X. Associations of vitamin D deficiency and vitamin D receptor (Cdx-2, Fok I, Bsm I and Taq I) polymorphisms with the risk of primary open-angle glaucoma. BMC Ophthalmol. 2016, 16, 116. [Google Scholar] [CrossRef] [Green Version]
- Kocaturk, T.; Bekmez, S.; Unubol, M. Effects of vitamin D deficiency on intraocular pressure values obtained by ocular response analyzer. Int. Ophthalmol. 2020, 40, 697–701. [Google Scholar] [CrossRef]
- Kutuzova, G.D.; Gabelt, B.T.; Kiland, J.A.; Hennes-Beann, E.A.; Kaufman, P.L.; DeLuca, H.F. 1α,25-Dihydroxyvitamin D3 and its analog, 2-methylene-19-nor-(20S)-1α,25-dihydroxyvitamin D3 (2MD), suppress intraocular pressure in non-human primates. Arch. Biochem. Biophys. 2012, 518, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Lazzara, F.; Amato, R.; Platania, C.B.M.; Conti, F.; Chou, T.H.; Porciatti, V.; Drago, F.; Bucolo, C. 1α,25-dihydroxyvitamin D3 protects retinal ganglion cells in glaucomatous mice. J. Neuroinflamm. 2021, 18, 206. [Google Scholar] [CrossRef]
- Ko, M.L.; Peng, P.H.; Hsu, S.Y.; Chen, C.F. Dietary deficiency of vitamin E aggravates retinal ganglion cell death in experimental glaucoma of rats. Curr. Eye Res. 2010, 35, 842–849. [Google Scholar] [CrossRef]
- Özmen, C.; Göçün, P.; Değim, Z.; Özkan, Y.; Onol, M.; Aktaş, Z. Retinal ganglion cell protection via topical and systemic alpha-tocopherol administration in optic nerve crush model of rat. Turk. J. Ophthalmol. 2013, 43, 161–166. [Google Scholar] [CrossRef]
- Yellanki, S.K.; Anna, B.; Kishan, M.R. Preparation and in vivo evaluation of sodium alginate-poly (vinyl alcohol) electrospun nanofibers of forskolin for glaucoma treatment. Pak. J. Pharm. Sci. 2019, 32, 669–674. [Google Scholar] [PubMed]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical curcumin nanocarriers are neuroprotective in eye disease. Sci. Rep. 2018, 8, 11066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.H.; Ko, Y.C.; Chang, Y.F.; Huang, S.H.; Liu, C.J. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Pandian, S.; Ponnusamy, C.; Palanichamy, R.; Muthusamy, S.; Kandasamy, R. Co-encapsulated resveratrol and quercetin in chitosan and peg modified chitosan nanoparticles: For efficient intra ocular pressure reduction. Int. J. Biol. Macromol. 2017, 104, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Research Guidelines for Evaluating the Safety and Efficacy of Herbal Medicines; World Health Organization: Geneva, Switzerland, 1993; p. 89. [Google Scholar]
- Suntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Vetrugno, M.; Uva, M.G.; Russo, V.; Iester, M.; Ciancaglini, M.; Brusini, P.; Centofanti, M.; Rossetti, L.M. Oral administration of forskolin and rutin contributes to intraocular pressure control in primary open angle glaucoma patients under maximum tolerated medical therapy. J. Ocul. Pharmacol. Ther. 2012, 28, 536–541. [Google Scholar] [CrossRef]
- Nebbioso, M.; Rusciano, D.; Pucci, B.; Zicari, A.M.; Grenga, R.; Pescocolido, N. Treatment of glaucomatous patients by means of food supplement to reduce the ocular discomfort: A double blind randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1117–1122. [Google Scholar]
- Nebbioso, M.; Belcaro, G.; Librando, A.; Rusciano, D.; Steigerwalt, R.D., Jr.; Pescosolido, N. Forskolin and rutin prevent intraocular pressure spikes after Nd:YAG laser iridotomy. Panminerva Med. 2012, 54, 77–82. [Google Scholar]
- Mutolo, M.G.; Albanese, G.; Rusciano, D.; Pescosolido, N. Oral Administration of forskolin, homotaurine, carnosine, and folic acid in patients with primary open angle glaucoma: Changes in intraocular pressure, pattern electroretinogram amplitude, and foveal sensitivity. J. Ocul. Pharmacol. Ther. 2016, 32, 178–183. [Google Scholar] [CrossRef]
- Rolle, T.; Dallorto, L.; Rossatto, S.; Curto, D.; Nuzzi, R. Assessing the Performance of daily intake of a homotaurine, carnosine, forskolin, vitamin B2, vitamin B6, and magnesium based food supplement for the maintenance of visual function in patients with primary open angle glaucoma. J. Ophthalmol. 2020, 2020, 7879436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, K.; Kaidzu, S.; Tsutsui, A.; Mochiji, M.; Matsuoka, Y.; Takagi, Y.; Miyamoto, E.; Tanito, M. Effects of French maritime pine bark/bilberry fruit extracts on intraocular pressure for primary open-angle glaucoma. J. Clin. Biochem. Nutr. 2021, 68, 67–72. [Google Scholar] [CrossRef]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef]
- Gupta, R.; Patil, B.; Shah, B.M.; Bali, S.J.; Mishra, S.K.; Dada, T. Evaluating eye drop instillation technique in glaucoma patients. J. Glaucoma 2012, 21, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Rahic, O.; Tucak, A.; Omerovic, N.; Sirbubalo, M.; Hindija, L.; Hadziabdic, J.; Vranic, E. Novel drug delivery systems fighting glaucoma: Formulation obstacles and solutions. Pharmaceutics 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, X.; Yang, Y.; Zhang, L.; Liu, R.; Li, Z. Trimethyl chitosan nanoparticles for ocular baicalein delivery: Preparation, optimization, in vitro evaluation, in vivo pharmacokinetic study and molecular dynamics simulation. Int. J. Biol. Macromol. 2020, 156, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.T.; Youle, R.J. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997, 272, 13829–13834. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.T.; Youle, R.J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998, 273, 10777–10783. [Google Scholar] [CrossRef] [Green Version]
- Levin, L.A.; Schlamp, C.L.; Spieldoch, R.L.; Geszvain, K.M.; Nickells, R.W. Identification of the bcl-2 family of genes in the rat retina. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2545–2553. [Google Scholar]
- Chen, S.T.; Garey, L.J.; Jen, L.S. Bcl-2 proto-oncogene protein immunoreactivity in normally developing and axotomised rat retinas. Neurosci. Lett. 1994, 172, 11–14. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients 2022, 14, 534. https://doi.org/10.3390/nu14030534
Sim RH, Sirasanagandla SR, Das S, Teoh SL. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients. 2022; 14(3):534. https://doi.org/10.3390/nu14030534
Chicago/Turabian StyleSim, Ru Hui, Srinivasa Rao Sirasanagandla, Srijit Das, and Seong Lin Teoh. 2022. "Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update" Nutrients 14, no. 3: 534. https://doi.org/10.3390/nu14030534
APA StyleSim, R. H., Sirasanagandla, S. R., Das, S., & Teoh, S. L. (2022). Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients, 14(3), 534. https://doi.org/10.3390/nu14030534