Insights into the Research Trends on Bovine Colostrum: Beneficial Health Perspectives with Special Reference to Manufacturing of Functional Foods and Feed Supplements
Abstract
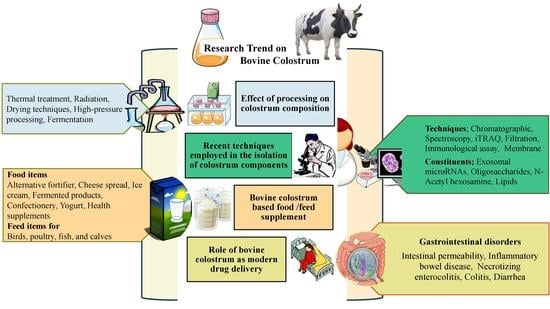
:1. Introduction
2. Impact of Processing Techniques and Conditions on Colostrum Constituents
Other Aspects That Might Influence the Composition of Bovine Colostrum
3. Recent Advancements in the Isolation and Identification of Novel BC Components
4. Bovine Colostrum as Modern Deliverable Nutraceutical Formulations
4.1. Food Supplement
4.2. Feed Supplement
5. Current Evidence of Therapeutic Use of BC
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehra, R.; Singh, R.; Nayan, V.; Buttar, H.S.; Kumar, N.; Kumar, S.; Bhardwaj, A.; Kaushik, R.; Kumar, H. Nutritional Attributes of Bovine Colostrum Components in Human Health and Disease: A Comprehensive Review. Food Biosci. 2021, 40, 100907. [Google Scholar] [CrossRef]
- Bagwe-Parab, S.; Yadav, P.; Kaur, G.; Tuli, H.S.; Buttar, H.S. Therapeutic Applications of Human and Bovine Colostrum in the Treatment of Gastrointestinal Diseases and Distinctive Cancer Types: The Current Evidence. Front. Pharmacol. 2020, 11, 01100. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Kang, S.; Cao, X.; Zheng, Y.; Wu, J.; Wu, R.; Shao, J.; Yang, M.; Yue, X. Characterization and Comparison of Lipids in Bovine Colostrum and Mature Milk Based on UHPLC-QTOF-MS Lipidomics. Food Res. Int. 2020, 136, 109490. [Google Scholar] [CrossRef] [PubMed]
- Feeney, S.; Morrin, S.T.; Joshi, L.; Hickey, R.M. The Role of Immunoglobulins from Bovine Colostrum and Milk in Human Health Promotion. In Novel Proteins for Food, Pharmaceuticals and Agriculture; Hayes, M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 291–314. ISBN 978-1-119-38533-2. [Google Scholar]
- Hege, J.; Ghebremedhin, M.; Joshi, B.L.; Schreiber, C.; Vilgis, T.A. Soft Gels from Bovine Colostrum. Int. J. Gastron. Food Sci. 2021, 23, 100278. [Google Scholar] [CrossRef]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Immunoglobulin G Content and Colostrum Composition of Different Goat and Sheep Breeds in Switzerland and Germany. J. Dairy Sci. 2019, 102, 5542–5549. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Puppel, K.; Gołębiewski, M.; Grodkowski, G.; Slósarz, J.; Kunowska-Slósarz, M.; Solarczyk, P.; Łukasiewicz, M.; Balcerak, M.; Przysucha, T. Composition and Factors Affecting Quality of Bovine Colostrum: A Review. Animals 2019, 9, 1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk Derived Antimicrobial Bioactive Peptides: A Review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Johnson, S.K.; Busetti, F.; Solah, V.A. Formation and Degradation of Beta-Casomorphins in Dairy Processing. Crit. Rev. Food Sci. Nutr. 2015, 55, 1955–1967. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive Peptides Derived from Bovine Whey Proteins. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Jarmołowska, B.; Sidor, K.; Iwan, M.; Bielikowicz, K.; Kaczmarski, M.; Kostyra, E.; Kostyra, H. Changes of β-Casomorphin Content in Human Milk during Lactation. Peptides 2007, 28, 1982–1986. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Currie, A.J.; Ren, S.; Bering, S.B.; Sangild, P.T. Heat Treatment and Irradiation Reduce Anti-Bacterial and Immune-Modulatory Properties of Bovine Colostrum. J. Funct. Foods 2019, 57, 182–189. [Google Scholar] [CrossRef]
- Butler, J.E. Bovine Immunoglobulins: A Review. J. Dairy Sci. 1969, 52, 1895–1909. [Google Scholar] [CrossRef]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune Components of Bovine Colostrum and Milk. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG Placental Transfer in Healthy and Pathological Pregnancies. Clin. Dev. Immunol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Gapper, L.W.; Copestake, D.E.J.; Otter, D.E.; Indyk, H.E. Analysis of Bovine Immunoglobulin G in Milk, Colostrum and Dietary Supplements: A Review. Anal. Bioanal. Chem. 2007, 389, 93–109. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Stankevicius, A.; Grigas, J.; Pautienius, A.; Bernatoniene, J.; Jakstas, V.; et al. Fermented, Ultrasonicated, and Dehydrated Bovine Colostrum: Changes in Antimicrobial Properties and Immunoglobulin Content. J. Dairy Sci. 2020, 103, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Borad, S.G.; Singh, A.K.; Meena, G.S.; Arora, S.; Raju, P.N.; Sabikhi, L. Optimization of Spray Drying of Colostrum Protein Ingredients—A Rheological Approach. J. Food Eng. 2021, 288, 110247. [Google Scholar] [CrossRef]
- Cao, X.; Yang, M.; Yang, N.; Liang, X.; Tao, D.; Liu, B.; Wu, J.; Yue, X. Characterization and Comparison of Whey N-Glycoproteomes from Human and Bovine Colostrum and Mature Milk. Food Chem. 2019, 276, 266–273. [Google Scholar] [CrossRef]
- Elsohaby, I.; McClure, J.T.; Cameron, M.; Heider, L.C.; Keefe, G.P. Rapid Assessment of Bovine Colostrum Quality: How Reliable Are Transmission Infrared Spectroscopy and Digital and Optical Refractometers? J. Dairy Sci. 2017, 100, 1427–1435. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, S. Identification and Comparison of Exosomal MicroRNAs in the Milk and Colostrum of Two Different Cow Breeds. Gene 2020, 743, 144609. [Google Scholar] [CrossRef] [PubMed]
- Urakami, H.; Saeki, M.; Watanabe, Y.; Kawamura, R.; Nishizawa, S.; Suzuki, Y.; Watanabe, A.; Ajisaka, K. Isolation and Assessment of Acidic and Neutral Oligosaccharides from Goat Milk and Bovine Colostrum for Use as Ingredients of Infant Formulae. Int. Dairy J. 2018, 83, 1–9. [Google Scholar] [CrossRef]
- Dzik, S.; Miciński, B.; Aitzhanova, I.; Miciński, J.; Pogorzelska, J.; Beisenov, A.; Kowalski, I.M. Properties of Bovine Colostrum and the Possibilities of Use. Pol. Ann. Med. 2017, 24, 295–299. [Google Scholar] [CrossRef]
- Juhl, S.M.; Ye, X.; Zhou, P.; Li, Y.; Iyore, E.O.; Zhang, L.; Jiang, P.; van Goudoever, J.B.; Greisen, G.; Sangild, P.T. Bovine Colostrum for Preterm Infants in the First Days of Life: A Randomized Controlled Pilot Trial. J. Pediatric Gastroenterol. Nutr. 2018, 66, 471–478. [Google Scholar] [CrossRef]
- da Cruz, T.M.P.; Moretti, D.B.; Nordi, W.M.; Cyrino, J.E.P.; Machado-Neto, R. Dietary Lyophilized Colostrum Alters Distribution of Goblet Cells and the Intestinal Epithelium of Piaractus Mesopotamicus. Aquaculture 2017, 468, 286–292. [Google Scholar] [CrossRef]
- Afzal, I.; Khan, A.A.; Khaliq, T.; Hamadani, H.; Shafi, M.; Raja, T.A. Effect of Bovine Colostrum Supplemented Diets on Performance of Broiler Chicken. Indian J. Poult. Sci. 2017, 52, 157. [Google Scholar] [CrossRef]
- Akdemir, F.; Bayril, T.; Baran, M.S.; Yildiz, A.S.; Kahraman, M.; Orhan, C.; Sahin, K. The Effect of Dietary Colostrum Powder on Performance, Carcass Yields and Serum Lipid Peroxidation Levels in Japanese Quails (Coturnix Coturnix Japonica). J. Appl. Anim. Res. 2018, 46, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Alsayed, A.; Al-Doori, A.; Al-Dulaimi, A.; Alnaseri, A.; Abuhashish, J.; Aliasin, K.; Alfayoumi, I. Influences of Bovine Colostrum on Nasal Swab Microbiome and Viral Upper Respiratory Tract Infections—A Case Report. Respir. Med. Case Rep. 2020, 31, 101189. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot Study of Probiotic/Colostrum Supplementation on Gut Function in Children with Autism and Gastrointestinal Symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Borad, S.G.; Singh, A.K. Colostrum Immunoglobulins: Processing, Preservation and Application Aspects. Int. Dairy J. 2018, 85, 201–210. [Google Scholar] [CrossRef]
- Gomes, R.D.S.; Anaya, K.; Galdino, A.B.S.; Oliveira, J.P.F.; Gama, M.A.S.; Medeiros, C.A.C.X.; Gavioli, E.C.; Porto, A.L.F.; Rangel, A.H.N. Bovine Colostrum: A Source of Bioactive Compounds for Prevention and Treatment of Gastrointestinal Disorders. NFS J. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Harish, K.; Naveen, K.; Raman, S.; Arun, G.; Chand, R. Effect of Heat Treatments of Goat Colostrum on Bacterial Counts, Viscosity, and Immunoglobulin G Concentration. Indian J. Dairy Sci. 2015, 68, 132–136. [Google Scholar]
- Kumar, H.; Devbrat; Kumar, N.; Garg, V.; Seth, R.; Kumar, B.S.B. Sialic Acid Content in Colostrum of Two Cross Breed Dairy Goat: Effect of Breed and Lactation. J. Anim. Res. 2015, 5, 785. [Google Scholar] [CrossRef]
- Elfstrand, L.; Lindmark-Månsson, H.; Paulsson, M.; Nyberg, L.; Åkesson, B. Immunoglobulins, Growth Factors and Growth Hormone in Bovine Colostrum and the Effects of Processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Foster, D.M.; Poulsen, K.P.; Sylvester, H.J.; Jacob, M.E.; Casulli, K.E.; Farkas, B.E. Effect of High-Pressure Processing of Bovine Colostrum on Immunoglobulin G Concentration, Pathogens, Viscosity, and Transfer of Passive Immunity to Calves. J. Dairy Sci. 2016, 99, 8575–8588. [Google Scholar] [CrossRef] [Green Version]
- Salar, S.; Jafarian, S.; Mortazavi, A.; Nasiraie, L.R. Effect of Hurdle Technology of Gentle Pasteurisation and Drying Process on Bioactive Proteins, Antioxidant Activity and Microbial Quality of Cow and Buffalo Colostrum. Int. Dairy J. 2021, 121, 105138. [Google Scholar] [CrossRef]
- Borad, S.G.; Singh, A.K.; Kapila, S.; Behare, P.; Arora, S.; Sabikhi, L. Influence of Unit Operations on Immunoglobulins and Thermal Stability of Colostrum Fractions. Int. Dairy J. 2019, 93, 85–91. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Aagaard, S.; Hesselballe Hansen, T.; Nguyen, D.N.; De Gobba, C.; Lametsch, R.; Sangild, P.T. Bioactive Proteins in Bovine Colostrum and Effects of Heating, Drying and Irradiation. Food Funct. 2020, 11, 2309–2327. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, R.; Zhang, J.; Zhou, P. Heat-Induced Denaturation and Bioactivity Changes of Whey Proteins. Int. Dairy J. 2021, 123, 105175. [Google Scholar] [CrossRef]
- Elizondo-Salazar, J.A.; Jayarao, B.M.; Heinrichs, A.J. Effect of Heat Treatment of Bovine Colostrum on Bacterial Counts, Viscosity, and Immunoglobulin G Concentration. J. Dairy Sci. 2010, 93, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Mann, S.; Curone, G.; Chandler, T.L.; Moroni, P.; Cha, J.; Bhawal, R.; Zhang, S. Heat Treatment of Bovine Colostrum: I. Effects on Bacterial and Somatic Cell Counts, Immunoglobulin, Insulin, and IGF-I Concentrations, as Well as the Colostrum Proteome. J. Dairy Sci. 2020, 103, 9368–9383. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Q.; Bomser, J.A.; Zhang, Q.H. Effects of Pulsed Electric Fields and Heat Treatment on Stability and Secondary Structure of Bovine Immunoglobulin G. J. Agric. Food Chem. 2005, 53, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Characteristics of Bovine Lactoferrin Powders Produced through Spray and Freeze Drying Processes. Int. J. Biol. Macromol. 2017, 95, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Boggs, I.; Weeks, M.; Li, Q.; Wu, H.; Harris, P.; Ma, Y.; Day, L. Kinetic Modelling of the Heat Stability of Bovine Lactoferrin in Raw Whole Milk. J. Food Eng. 2020, 280, 109977. [Google Scholar] [CrossRef]
- Lomónaco, M.; Sowul, M.; Gutiérrez, G.; Malacari, D.; Álvarez, I.; Porta, N.G.; Zabal, O.; Trono, K. Efficacy of the Spray-Drying Treatment to Inactivate the Bovine Leukemia Virus in Bovine Colostrum. J. Dairy Sci. 2020, 103, 6504–6510. [Google Scholar] [CrossRef]
- Birwal, P.; Deshmukh, G.P.; Ravindra, M.R. Nonthermal Processing of Dairy Beverages. In Milk-Based Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–426. ISBN 978-0-12-815504-2. [Google Scholar]
- Gosch, T.; Apprich, S.; Kneifel, W.; Novalin, S. A Combination of Microfiltration and High Pressure Treatment for the Elimination of Bacteria in Bovine Colostrum. Int. Dairy J. 2014, 34, 41–46. [Google Scholar] [CrossRef]
- Inabu, Y.; Yamamoto, H.; Yamano, H.; Taguchi, Y.; Okada, S.; Etoh, T.; Shiotsuka, Y.; Fujino, R.; Takahashi, H. Glucagon-like Peptide 2 (GLP-2) in Bovine Colostrum and Transition Milk. Heliyon 2021, 7, e07046. [Google Scholar] [CrossRef]
- Robbers, L.; Jorritsma, R.; Nielen, M.; Koets, A. A Scoping Review of On-Farm Colostrum Management Practices for Optimal Transfer of Immunity in Dairy Calves. Front. Vet. Sci. 2021, 8, 668639. [Google Scholar] [CrossRef]
- Jones, L.R.; Taylor, A.W.; Hines, H.C. Characteristics of Frozen Colostrum Thawed in a Microwave Oven. J. Dairy Sci. 1987, 70, 1941–1945. [Google Scholar] [CrossRef]
- Mehra, R.; Kumar, S.; Verma, N.; Kumar, N.; Singh, R.; Bhardwaj, A.; Nayan, V.; Kumar, H. Chemometric Approaches to Analyze the Colostrum Physicochemical and Immunological (IgG) Properties in the Recently Registered Himachali Pahari Cow Breed in India. LWT 2021, 145, 111256. [Google Scholar] [CrossRef]
- Xin, H.; Xu, Y.; Chen, Y.; Chen, G.; Steele, M.A.; Guan, L.L. Short Communication: Odd-Chain and Branched-Chain Fatty Acid Concentrations in Bovine Colostrum and Transition Milk and Their Stability under Heating and Freezing Treatments. J. Dairy Sci. 2020, 103, 11483–11489. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; O’Donovan, M.; Murphy, J.P.; Sugrue, K.; Mannion, D.; McCarthy, W.P.; Timlin, M.; Kilcawley, K.N.; Hickey, R.M.; Tobin, J.T. Evolution of the Bovine Milk Fatty Acid Profile—From Colostrum to Milk Five Days Post Parturition. Int. Dairy J. 2020, 104, 104655. [Google Scholar] [CrossRef]
- Wąsowska, E.; Puppel, K. Changes in the Content of Immunostimulating Components of Colostrum Obtained from Dairy Cows at Different Levels of Production: Immunostimulating Components of Colostrum Obtained from Dairy Cows. J. Sci. Food Agric. 2018, 98, 5062–5068. [Google Scholar] [CrossRef]
- Hyrslova, I.; Krausova, G.; Michlova, T.; Kana, A.; Curda, L. Fermentation Ability of Bovine Colostrum by Different Probiotic Strains. Fermentation 2020, 6, 93. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Ikkere, L.E.; Pugajeva, I.; Zavistanaviciute, P.; Lele, V.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Klupsaite, D.; et al. The Effects of Ultrasonication, Fermentation with Lactobacillus Sp., and Dehydration on the Chemical Composition and Microbial Contamination of Bovine Colostrum. J. Dairy Sci. 2018, 101, 6787–6798. [Google Scholar] [CrossRef]
- Cummins, C.; Lorenz, I.; Kennedy, E. Short Communication: The Effect of Storage Conditions over Time on Bovine Colostral Immunoglobulin G Concentration, Bacteria, and PH. J. Dairy Sci. 2016, 99, 4857–4863. [Google Scholar] [CrossRef]
- Zheng, J.; Xi, C.; Wang, G.; Cao, S.; Tang, B.; Mu, Z. Simultaneous Determination of 20 Antibiotics in Bovine Colostrum Tablet Using UHPLC—MS/MS and SPE. Chromatographia 2018, 81, 947–957. [Google Scholar] [CrossRef]
- An, C.; Yan, X.; Lu, C.; Zhu, X. Effect of Spectral Pretreatment on Qualitative Identification of Adulterated Bovine Colostrum by Near-Infrared Spectroscopy. Infrared Phys. Technol. 2021, 118, 103869. [Google Scholar] [CrossRef]
- Lee, H.; Nobrega de Moura Bell, J.M.L.; Barile, D. Discovery of Novel High-Molecular Weight Oligosaccharides Containing N -Acetylhexosamine in Bovine Colostrum Whey Permeate Hydrolyzed with Aspergillus Oryzae β-Galactosidase. J. Agric. Food Chem. 2019, 67, 3313–3322. [Google Scholar] [CrossRef]
- Skalka, V.; Shakhno, N.; Ečer, J.; Čurda, L. Separation of Immunoglobulins from Colostrum Using Methods Based on Salting-out Techniques. Czech J. Food Sci. 2017, 35, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.L.; Barile, D.; Liu, Y.; de Moura Bell, J.M.L.N. Role of pH in the Recovery of Bovine Milk Oligosaccharides from Colostrum Whey Permeate by Nanofiltration. Int. Dairy J. 2017, 66, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Cao, X.; Wu, R.; Liu, B.; Ye, W.; Yue, X.; Wu, J. Comparative Proteomic Exploration of Whey Proteins in Human and Bovine Colostrum and Mature Milk Using ITRAQ-Coupled LC-MS/MS. Int. J. Food Sci. Nutr. 2017, 68, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Sha, M.; Wang, H.-Y. Laser Perturbation Two-dimensional Correlation Raman Spectroscopy for Quality Control of Bovine Colostrum Products. J. Raman Spectrosc. 2017, 48, 1111–1115. [Google Scholar] [CrossRef]
- Drikic, M.; Windeyer, C.; Olsen, S.; Fu, Y.; Doepel, L.; De Buck, J. Determining the IgG Concentrations in Bovine Colostrum and Calf Sera with a Novel Enzymatic Assay. J. Anim. Sci. Biotechnol. 2018, 9, 69. [Google Scholar] [CrossRef]
- Urtasun, N.; Baieli, M.F.; Hirsch, D.B.; Martínez-Ceron, M.C.; Cascone, O.; Wolman, F.J. Lactoperoxidase Purification from Whey by Using Dye Affinity Chromatography. Food Bioprod. Process. 2017, 103, 58–65. [Google Scholar] [CrossRef]
- Heidebrecht, H.-J.; Kainz, B.; Schopf, R.; Godl, K.; Karcier, Z.; Kulozik, U.; Förster, B. Isolation of Biofunctional Bovine Immunoglobulin G from Milk—And Colostral Whey with Mixed-Mode Chromatography at Lab and Pilot Scale. J. Chromatogr. A 2018, 1562, 59–68. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Martins, J.T.; Pinheiro, A.C.; Madalena, D.A.; Marques, A.; Nunes, R.; Vicente, A.A. Nanoparticles of Lactoferrin for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–168. ISBN 978-0-12-815663-6. [Google Scholar]
- Silva, E.G.d.S.O.; Rangel, A.H.d.N.; Mürmam, L.; Bezerra, M.F.; Oliveira, J.P.F.d. Bovine Colostrum: Benefits of Its Use in Human Food. Food Sci. Technol. 2019, 39, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Thiel, A.; Glávits, R.; Murbach, T.S.; Endres, J.R.; Reddeman, R.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. Toxicological Evaluations of Colostrum Ultrafiltrate. Regul. Toxicol. Pharmacol. 2019, 104, 39–49. [Google Scholar] [CrossRef]
- Davis, P.F.; Greenhill, N.S.; Rowan, A.M.; Schollum, L.M. The Safety of New Zealand Bovine Colostrum: Nutritional and Physiological Evaluation in Rats. Food Chem. Toxicol. 2007, 45, 229–236. [Google Scholar] [CrossRef]
- Buttar, H.S.; Bagwe, S.M.; Bhullar, S.K.; Kaur, G. Health Benefits of Bovine Colostrum in Children and Adults. In Dairy in Human Health and Disease Across the Lifespan; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–20. ISBN 978-0-12-809868-4. [Google Scholar]
- Pieper, R.; Scharek-Tedin, L.; Zetzsche, A.; Röhe, I.; Kröger, S.; Vahjen, W.; Zentek, J. Bovine Milk—Based Formula Leads to Early Maturation-like Morphological, Immunological, and Functional Changes in the Jejunum of Neonatal Piglets. J. Anim. Sci. 2016, 94, 989–999. [Google Scholar] [CrossRef] [Green Version]
- Pluske, J.R. Invited Review: Aspects of Gastrointestinal Tract Growth and Maturation in the Pre—And Postweaning Period of Pigs. J. Anim. Sci. 2016, 94, 399–411. [Google Scholar] [CrossRef] [Green Version]
- Chae, A.; Aitchison, A.; Day, A.S.; Keenan, J.I. Bovine Colostrum Demonstrates Anti-Inflammatory and Antibacterial Activity in In Vitro Models of Intestinal Inflammation and Infection. J. Funct. Foods 2017, 28, 293–298. [Google Scholar] [CrossRef]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef]
- Pammi, M.; Abrams, S.A. Oral Lactoferrin for the Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2015; p. 007137. [Google Scholar]
- Batista da Silva Galdino, A.; do Nascimento Rangel, A.H.; Buttar, H.S.; Sales Lima Nascimento, M.; Cristina Gavioli, E.; Oliveira, R.d.P.; Cavalcanti Sales, D.; Urbano, S.A.; Anaya, K. Bovine Colostrum: Benefits for the Human Respiratory System and Potential Contributions for Clinical Management of COVID-19. Food Agric. Immunol. 2021, 32, 143–162. [Google Scholar] [CrossRef]
- Khan, Z.; Macdonald, C.; Wicks, A.C.; Holt, M.P.; Floyd, D.; Ghosh, S.; Wright, N.A.; Playford, R.J. Use of the ‘Nutriceutical’, Bovine Colostrum, for the Treatment of Distal Colitis: Results from an Initial Study: Colostrum Enemas for Distal Colitis. Aliment. Pharmacol. Ther. 2002, 16, 1917–1922. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Pan, X.; Nguyen, D.N.; Brunse, A.; Bojesen, A.M.; Rudloff, S.; Mortensen, M.S.; Burrin, D.G.; Sangild, P.T. Human Milk Fortification with Bovine Colostrum Is Superior to Formula-Based Fortifiers to Prevent Gut Dysfunction, Necrotizing Enterocolitis, and Systemic Infection in Preterm Pigs. J. Parenter. Enter. Nutr. 2019, 43, 252–262. [Google Scholar] [CrossRef]
- Li, Y.; Juhl, S.M.; Ye, X.; Shen, R.L.; Iyore, E.O.; Dai, Y.; Sangild, P.T.; Greisen, G.O. A Stepwise, Pilot Study of Bovine Colostrum to Supplement the First Enteral Feeding in Preterm Infants (Precolos): Study Protocol and Initial Results. Front Pediatr 2017, 5, 42. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Olin, A.B.; Nguyen, D.N. Fortification with Bovine Colostrum Enhances Antibacterial Activity of Human Milk. J. Parenter. Enter. Nutr. 2021, 45, 1417–1424. [Google Scholar] [CrossRef]
- Reyes-Portillo, K.A.; Quintero-Lira, A.; Piloni-Martini, J.; Fajardo-Espinoza, F.S.; Hernández-Sánchez, H.; Soto-Simental, S. Using BAMLET Complex in a Functional Spreadable Cheese Elaborated with Bovine Colostrum. J. Food Sci. Technol. 2021, 2021. 58, 3465–3472. [Google Scholar] [CrossRef]
- Saleh, A.; Moussa, M.; Hassabu, E.; Ewis, A. The Use of Colostrum to Improve the Functional and Chemical Properties of Ice Cream. J. Product. Dev. 2020, 25, 363–375. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of Antimicrobial Gummy Candies with Addition of Bovine Colostrum, Essential Oils and Probiotics. Int. J. Food Sci. Technol. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- Cotârleţ, M.; Vasile, A.M.; Gaspar-Pintiliescu, A.; Oancea, A.; Bahrim, G.E. Tribiotication Strategy for the Functionalization of Bovine Colostrum through the Biochemical Activities of Artisanal and Selected Starter Cultures. CyTA—J. Food 2020, 18, 274–280. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.S.; Zaki, D.A. Production of Novel Functional Yoghurt Fortified with Bovine Colostrum and Date Syrup for Children. Alex. Sci. Exch. J. 2018, 39, 651–662. [Google Scholar] [CrossRef] [Green Version]
- El-Loly, M.M.; Hassan, L.K.; Farahat, E.S.A. Impact of Heat Treatments and Some Technological Processing on Immunoglobulins of Egyptian Buffalo’s Milk. Int. J. Biol. Macromol. 2019, 123, 939–944. [Google Scholar] [CrossRef]
- Rathe, M.; Müller, K.; Sangild, P.T.; Husby, S. Clinical Applications of Bovine Colostrum Therapy: A Systematic Review. Nutr Rev 2014, 72, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Marnila, P.; Korhonen, H. Milk Immunoglobulins for Health Promotion. Int. Dairy J. 2006, 16, 1262–1271. [Google Scholar] [CrossRef]
- Sydney, A.C.N.; Ikeda, I.K.; de Oliveira Ribeiro, M.C.; Sydney, E.B.; de Carvalho Neto, D.P.; Karp, S.G.; Rodrigues, C.; Soccol, C.R. Colostrum New Insights: Products and Processes. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 397–422. ISBN 978-0-12-823506-5. [Google Scholar]
- Tay, N.; Tan, Y.C.; Chng, K.; Libedinsky, C.; Gluckman, P.; Buschdorf, J.P. Effect of Human Milk Formula with Bovine Colostrum Supplementation on Bone Mineral Density in Infant Cynomolgus Macaques. J. Dev. Orig. Health Dis. 2018, 9, 172–181. [Google Scholar] [CrossRef] [PubMed]
- El-Loly, M.M. Bovine Milk Immunoglobulins in Relation to Human Health. Int. J. Dairy Sci. 2007, 2, 183–195. [Google Scholar] [CrossRef]
- McLeod, K.H.; Richards, J.L.; Yap, Y.A.; Mariño, E. Dietary Short Chain Fatty Acids: How the Gut Microbiota Fight Against Autoimmune and Inflammatory Diseases. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–159. ISBN 978-0-12-813820-5. [Google Scholar]
- Hałasa, M.; Maciejewska-Markiewicz, D.; Baśkiewicz-Hałasa, M.; Safranow, K.; Stachowska, E. Post-Delivery Milking Delay Influence on the Effect of Oral Supplementation with Bovine Colostrum as Measured with Intestinal Permeability Test. Medicina 2020, 56, 495. [Google Scholar] [CrossRef]
- Eslamian, G.; Ardehali, S.H.; Baghestani, A.-R.; Shariatpanahi, Z.V. Effects of Early Enteral Bovine Colostrum Supplementation on Intestinal Permeability in Critically Ill Patients: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrition 2019, 60, 106–111. [Google Scholar] [CrossRef]
- Barakat, S.H.; Meheissen, M.A.; Omar, O.M.; Elbana, D.A. Bovine Colostrum in the Treatment of Acute Diarrhea in Children: A Double-Blinded Randomized Controlled Trial. J. Trop. Pediatr. 2020, 66, 46–55. [Google Scholar] [CrossRef]
- Florén, C.-H.; Chinenye, S.; Elfstrand, L.; Hagman, C.; Ihse, I. ColoPlus, a New Product Based on Bovine Colostrum, Alleviates HIV-Associated Diarrhoea. Scand. J. Gastroenterol. 2006, 41, 682–686. [Google Scholar] [CrossRef]
- Nederend, M.; van Stigt, A.H.; Jansen, J.H.M.; Jacobino, S.R.; Brugman, S.; de Haan, C.A.M.; Bont, L.J.; van Neerven, R.J.J.; Leusen, J.H.W. Bovine IgG Prevents Experimental Infection with RSV and Facilitates Human T Cell Responses to RSV. Front. Immunol. 2020, 11, 1701. [Google Scholar] [CrossRef]
- Konishi, M.; Ishizaki, H.; Kameyama, K.; Murakami, K.; Yamamoto, T. The Effectiveness of Colostral Antibodies for Preventing Bovine Leukemia Virus (BLV) Infection in Vitro. BMC Vet. Res. 2018, 14, 419. [Google Scholar] [CrossRef]
- Morrin, S.T.; McCarthy, G.; Kennedy, D.; Marotta, M.; Irwin, J.A.; Hickey, R.M. Immunoglobulin G from Bovine Milk Primes Intestinal Epithelial Cells for Increased Colonization of Bifidobacteria. AMB Express 2020, 10, 114. [Google Scholar] [CrossRef]
- Oloroso-Chavez, K.; Andaya, P.; Wong, C. OR082 Bovine Colostrum Supplementation in Respiratory Allergies According to Sensitization: Subgroup Analysis of Randomized Controlled Trial. Ann. Allergy Asthma Immunol. 2017, 119, S11–S12. [Google Scholar] [CrossRef]
- Schiavi, M.C.; Di Tucci, C.; Colagiovanni, V.; Faiano, P.; Giannini, A.; D’Oria, O.; Prata, G.; Perniola, G.; Monti, M.; Zullo, M.A.; et al. A Medical Device Containing Purified Bovine Colostrum (M Onurelle B Iogel) in the Treatment of Vulvovaginal Atrophy in Postmenopausal Women: R Etrospective Analysis of Urinary Symptoms, Sexual Function, and Quality of Life. LUTS Low. Urin. Tract Symptoms 2019, 11, O11–O15. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-Pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef] [PubMed]
- Taruni R, T.; Sivaraman, M.; Dutta, T.; Dhanasekar, K.R. A Comparative Study to Evaluate the Efficacy of Oral Lactoferrin Fortified Bovine Colostrum with Oral Iron in the Treatment of Iron Deficiency Anemia. Int. J. Med. Public Health 2018, 8, 65–70. [Google Scholar] [CrossRef] [Green Version]
- El-Khawaga, A.; Abdelmaksoud, H. Effect of Lactoferrin Supplementation on Iron Deficiency Anemia in Primary School Children. Int. J. Med. Arts 2019, 1, 48–52. [Google Scholar] [CrossRef]
- Carvalho, C.A.M.; Casseb, S.M.M.; Gonçalves, R.B.; Silva, E.V.P.; Gomes, A.M.O.; Vasconcelos, P.F.C. Bovine Lactoferrin Activity against Chikungunya and Zika Viruses. J. Gen. Virol. 2017, 98, 1749–1754. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.-Z. Lactoferrin as Potential Preventative and Adjunct Treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Tanideh, N.; Abdordideh, E.; Yousefabad, S.L.A.; Daneshi, S.; Hosseinabadi, O.K.; Samani, S.M.; Derakhshan far, A. Evaluation of the Healing Effect of Honey and Colostrum in Treatment of Cutaneous Wound in Rat. Comp. Clin. Pathol. 2017, 26, 71–77. [Google Scholar] [CrossRef]
- Awad, H.A.; Imam, S.S.; Aboushady, N.M.; Ismail, R.I.H.; Abdou, R.M.; Azzam, N.T. Gut Priming with Oral Bovine Colostrum for Preterm Neonates: A Randomized Control Trial. QJM Int. J. Med. 2020, 113, hcaa063.025. [Google Scholar] [CrossRef]
- Bierut, T.; Duckworth, L.; Grabowsky, M.; Ordiz, M.I.; Laury, M.L.; Callaghan-Gillespie, M.; Maleta, K.; Manary, M.J. The Effect of Bovine Colostrum/Egg Supplementation Compared with Corn/Soy Flour in Young Malawian Children: A Randomized, Controlled Clinical Trial. Am. J. Clin. Nutr. 2021, 113, 420–427. [Google Scholar] [CrossRef]
- Kovacs, D.; Maresca, V.; Flori, E.; Mastrofrancesco, A.; Picardo, M.; Cardinali, G. Bovine Colostrum Induces the Differentiation of Human Primary Keratinocytes. FASEB J. 2020, 34, 6302–6321. [Google Scholar] [CrossRef] [Green Version]
- Fajardo-Espinoza, F.S.; Romero-Rojas, A.; Hernández-Sánchez, H.; Instituto Politécnico Nacional. Production of Bioactive Peptides from Bovine Colostrum Whey Using Enzymatic Hydrolysis. Rev. Mex. Ing. Química 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alinaghi, M.; Jiang, P.-P.; Brunse, A.; Sangild, P.; Bertram, H. Rapid Cerebral Metabolic Shift during Neonatal Sepsis Is Attenuated by Enteral Colostrum Supplementation in Preterm Pigs. Metabolites 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Sinn, D.H.; Gwak, G.-Y.; Kwon, Y.J.; Paik, S.W. Anti-Fibrotic Effect of Bovine Colostrum in Carbon Tetrachloride-Induced Hepatic Fibrosis. Precis. Future Med. 2017, 1, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Filipescu, I.E.; Leonardi, L.; Menchetti, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Piro, F.; Quattrone, A.; Barbato, O.; Brecchia, G. Preventive Effects of Bovine Colostrum Supplementation in TNBS-Induced Colitis in Mice. PLoS ONE 2018, 13, e0202929. [Google Scholar] [CrossRef]
- Funatogawa, K.; Tada, T.; Kuwahara-Arai, K.; Kirikae, T.; Takahashi, M. Enriched Bovine IgG Fraction Prevents Infections with Enterohaemorrhagic Escherichia Coli O157:H7, Salmonella Enterica Serovar Enteritidis, and Mycobacterium Avium. Food Sci. Nutr. 2019, 7, 2726–2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghirad, B.; Morgan, R.L.; Zeraatkar, D.; Zea, A.M.; Couban, R.; Johnston, B.C.; Florez, I.D. Human and Bovine Colostrum for Prevention of Necrotizing Enterocolitis: A Meta-Analysis. Pediatrics 2018, 142, e20180767. [Google Scholar] [CrossRef] [PubMed]
- McKenna, Z.; Berkemeier, Q.; Gorini, F.; Kuennen, M.; Naylor, A.; Kleint, A.; Gillum, T. Effects of Exercise in Hot and Humid Conditions and Bovine Colostrum on Salivary Immune Markers. J. Therm. Biol. 2020, 93, 102717. [Google Scholar] [CrossRef]

| Immunoglobins | |||
|---|---|---|---|
| Bovine (mg/mL) | Milk | Colostrum | Serum |
| IgG1 | 0.59 | 20–200 | 14.0 |
| IgG2 | 0.12 | 12.0 | 11.0 |
| IgM | 0.05 | 4.20 | 3.1 |
| IgA | 0.14 | 3.90 | 0.4 |
| Human (mg/mL) | |||
| Total IgG | 0.04 | 0.43 | 12.1 |
| IgM | 0.10 | 1.59 | 0.9 |
| IgA | 1.00 | 17.35 | 2.5 |
| Technique/Component | Study Aim | Subject/Sample | Key Findings | Reference |
|---|---|---|---|---|
| Isobaric tags for relative and absolute quantitation (iTRAQ)-coupled LC-MS | Proteomic analysis of whey protein in BC, mature milk, and human milk. | Colostrum (0–5 days) collected from Chinese Holsten (n = 30), mature milk (15–6 months), and human milk (n = 60). | iTRAQ-coupled LC-MS is the most advanced, reliable, and precise technique in the proteomic quantification of milk from different origins and lactation. | [64] |
| Laser-perturbation 2-D correlation Raman spectroscopy | Exploration of laser perturbation 2-D correlation Raman spectroscopy method for the reliable and rapid assessment of the quality of BC-based products. | Colostrum samples in milligrams were loaded in porous chips, followed by the Raman spectral analysis. The excitation wavelength was 785 nm and laser power was 450 nm. | The intended approach is simple, rapid (<5 min), and inexpensive.$$$The correlation coefficient analysis assists in improving the difference in experimental samples and improved spectral resolution.$$$In the future, this approach might be utilized to effectively differentiate milk powder in BC-based products. | [65] |
| UHPLC-QTOF-MS | Characterize and compare the lipids in mature milk and BC based on ultra-high-performance liquid chromatography–quadrupole time of flight mass spectroscopy (UHPLC-QTOF-MS) lipidomics. | Mature milk and BC. | A total of 335 lipids belonging to 13 subclasses were identified in both mature milk and BC.$$$n = 63 lipids were significantly different in mature milk and BC. Out of 63 significantly different lipids, SDLs (n = 21 SDLs) were found to be higher in BC; the rest (n = 42 SDLs) were found to be in an elevated concentration in mature milk. | [3] |
| Split trehalase immunoglobulin G assay (STIGA) | Develop a novel method for the estimation of IgG in BC and serum at the farm level. | BC (n = 60), calf serum (n = 83), and purified bovine IgG (12.8 mg/mL) used as standard. This STIGA is based on the enzymatic action of trehalase (TreA), which converts trehalose into glucose which is further detected by glucometer. | STIGA is a single step assay that requires less time as compared to other methods which directly measure IgG and could be a promising method to use for the detection of IgG at the farm level. | [66] |
| Dye affinity chromatography | Purification of lactoperoxidase (LPO) from whey by employing dye affinity chromatography. | Triazine dye (n = 18) was immobilized on Sepharose 6B, followed by the screening of their activity as possible ligands. | Dye–Sepharose (n = 5) matrix showed more than 90% adsorption of LPO without any pretreatment. The Reactive Red-4 Sepharose matrix can be used in the one-step purification of LPO from bovine whey.$$$The dye affinity chromatography has potential in the purification and recovery of LPO from bovine whey by using different chromatographic support. | [67] |
| Mixed-mode chromatography | Develop an efficient, cost-effective procedure to isolate pure IgG from colostrum whey with minimal activity loss. | Two modes were used to separate the IgG from colostrum whey. Capto multimodal chromatography material (MNC) and MEP HyperCel matrix were used to capture IgG. | The authors isolated pure IgG (130–150 g) from 3 L of whey in five hours. This mixed-mode chromatography results in the purity of 96.1% IgG. This technique can be used in the future for the pure, stable, and active isolation of IgG from bovine milk and colostrum. | [68] |
| Transmission infrared (IR) spectroscopy and Brix refractometer | Determine the effectiveness of transmission infrared spectroscopy (IR) and Brix refractometer (optical and digital) in the estimation of colostrum IgG concentration. | Colostrum samples of Holstein cow (n = 258). The concentration of IgG was determined in 255 samples using infrared spectroscopy (IR) and radial immunodiffusion assay (RID). Colostrum samples (n = 240) were analyzed by using a refractometer (optical and digital). | Transmission infrared (IR) spectroscopy is an accurate and rapid method to determine the colostrum quality, i.e., IgG, in lab-based testing where the Brix refractometer (digital and digital) is less effective. Moreover, the study suggests that transmission infrared spectroscopy is effective at the lab scale, while the Brix (digital and digital) can be used to determine the colostrum quality at the farm level. | [21] |
| Exosomal microRNAs | Identify and compare the exosomal $$$microRNAs in both milk and colostrum. | Dogu Anadolu Kirmizisi and Holstein cows, | A total of 795 miRNAs were expressed and identified differently, out of which 545 were identified as miRNAs, of which 250 were identified as novel miRNAs. These miRNAs regulate milk protein and fat metabolism. | [22] |
| Neutral and acidic oligosaccharides | Isolation of neutral and acidic oligosaccharides from BC and goat milk for infant formula. | 20 mL of BC (0–3 days) after parturition, human milk (n = 20) and goat milk were used for the isolation of oligosaccharides through the modified charcoal column method. | The oligosaccharides from goat milk have more potential in terms of adherence inhibition and safety to be utilized in infant formula. | [23] |
| N-glycoproteomes | Characterize and compare whey N-glycoproteomes from BC, HC, and mature milk. | A bioinformatics analysis using liquid chromatography (LC)–tandem mass spectrometry. Colostrum and mature milk were collected from Holstein cows (n = 60) and HC (n = 60). Further collected samples were divided into 3 pooled groups (n = 20). | N-Glycoproteomes were different in BC, HC, and mature milk. N-Glycoproteomes (68, 58, and 98) were identified in all milk samples. The composition of N-glycoproteomes is significantly changed with lactation. | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehra, R.; Garhwal, R.; Sangwan, K.; Guiné, R.P.F.; Lemos, E.T.; Buttar, H.S.; Visen, P.K.S.; Kumar, N.; Bhardwaj, A.; Kumar, H. Insights into the Research Trends on Bovine Colostrum: Beneficial Health Perspectives with Special Reference to Manufacturing of Functional Foods and Feed Supplements. Nutrients 2022, 14, 659. https://doi.org/10.3390/nu14030659
Mehra R, Garhwal R, Sangwan K, Guiné RPF, Lemos ET, Buttar HS, Visen PKS, Kumar N, Bhardwaj A, Kumar H. Insights into the Research Trends on Bovine Colostrum: Beneficial Health Perspectives with Special Reference to Manufacturing of Functional Foods and Feed Supplements. Nutrients. 2022; 14(3):659. https://doi.org/10.3390/nu14030659
Chicago/Turabian StyleMehra, Rahul, Renu Garhwal, Karnam Sangwan, Raquel P. F. Guiné, Edite Teixeira Lemos, Harpal Singh Buttar, Pradeep Kumar Singh Visen, Naveen Kumar, Anuradha Bhardwaj, and Harish Kumar. 2022. "Insights into the Research Trends on Bovine Colostrum: Beneficial Health Perspectives with Special Reference to Manufacturing of Functional Foods and Feed Supplements" Nutrients 14, no. 3: 659. https://doi.org/10.3390/nu14030659
APA StyleMehra, R., Garhwal, R., Sangwan, K., Guiné, R. P. F., Lemos, E. T., Buttar, H. S., Visen, P. K. S., Kumar, N., Bhardwaj, A., & Kumar, H. (2022). Insights into the Research Trends on Bovine Colostrum: Beneficial Health Perspectives with Special Reference to Manufacturing of Functional Foods and Feed Supplements. Nutrients, 14(3), 659. https://doi.org/10.3390/nu14030659