Dietary Linolenic Acid Increases Sensitizing and Eliciting Capacities of Cow’s Milk Whey Proteins in BALB/c Mice
Abstract
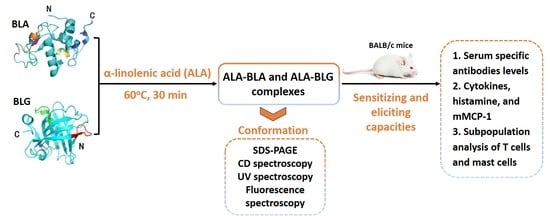
:1. Introduction
2. Materials and Methods
2.1. ALA–BLA/ALA–BLG Complex Preparation
2.2. ALA–BLA/ALA–BLG Complex Characterization
2.3. Animals and Experimental Design
2.4. Determination of IgE, IgG, IgG1, and IgG2a Antibodies Specific to BLA/BLG and ALA–BLA/ALA–BLG
2.5. PP, MLN, Spleen, and Peritoneal Cell Processing
2.6. In Vitro Cytokines, Histamine, and Mouse Mast Cell Protease-1 (mMCP-1) Measurement
2.7. Subpopulation Analysis of the T lymphocytes and Mast Cells by Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. ALA–BLA and ALA–BLG Complex Preparation and Characterization
3.2. Sensitizing and Eliciting Potential of ALA–BLA and ALA–BLG Complexes
3.3. Specificity of the Generated Antibodies
3.4. T-Cell and Cytokine Responses after ALA–BLA and ALA–BLG Complexes Challenge
3.5. Identification of Effector Cell Activation
3.6. ALA–BLA and ALA–BLG Complexes Modulated Treg Phenotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polloni, L.; Ferruzza, E.; Ronconi, L.; Toniolo, A.; Lazzarotto, F.; Bonaguro, R.; Celegato, N.; Muraro, A. Assessment of children’s nutritional attitudes before oral food challenges to identify patients at risk of food reintroduction failure: A prospective study. Allergy 2017, 72, 731–736. [Google Scholar] [CrossRef]
- Tordesillas, L.; Mondoulet, L.; Blazquez, A.B.; Benhamou, P.-H.; Sampson, H.A.; Berin, M.C. Epicutaneous immunotherapy induces gastrointestinal LAP + regulatory T cells and prevents food-induced anaphylaxis. J. Allergy Clin. Immunol. 2016, 139, 189–201.e4. [Google Scholar] [CrossRef] [Green Version]
- Yakaboski, E.; Robinson, L.; Arroyo, A.; Espinola, J.; Geller, R.; Sullivan, A.; Rudders, S.; Camargo, C. Early Introduction of Food Allergens and Risk of Developing Food Allergy. Nutrients 2021, 13, 2318. [Google Scholar] [CrossRef]
- Sokollik, C.; Carrard, A.; Rizzuti, D. Update on food allergy. Allergy 2015, 70, 1511–1520. [Google Scholar] [CrossRef]
- Dubois, A.E.J.; Turner, P.J.; Hourihane, J.; Ballmer-Weber, B.; Beyer, K.; Chan, C.H.; Gowland, M.H.; O’Hagan, S.; Regent, L.; Remington, B.; et al. How does dose impact on the severity of food-induced allergic reactions, and can this improve risk assessment for allergenic foods? Report from an ILSI Europe Food Allergy Task Force Expert Group and Workshop. Allergy 2018, 73, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Q.; Wang, Y.; Fu, L. Effect of Ultrasound Treatment on Allergenicity Reduction of Milk Casein via Colloid Formation. J. Agric. Food Chem. 2020, 68, 4678–4686. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Xu, L.; Li, S.; Costa, J.; Mafra, I.; Chen, G.; Gao, X.; Li, Z. Immunomodulatory Effect of Laccase/Caffeic Acid and Transglutaminase in Alleviating Shrimp Tropomyosin (Met e 1) Allergenicity. J. Agric. Food Chem. 2020, 68, 7765–7778. [Google Scholar] [CrossRef]
- Scheurer, S.; Toda, M.; Vieths, S. What makes an allergen? Clin. Exp. Allergy 2015, 45, 1150–1161. [Google Scholar] [CrossRef]
- Bublin, M.; Eiwegger, T.; Breiteneder, H. Do lipids influence the allergic sensitization process? J. Allergy Clin. Immunol. 2014, 134, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Y.; Tang, L.; de Villiers, W.J.; Cohen, D.; Woodward, J.; Finkelman, F.D.; Eckhardt, E.R. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J. Allergy Clin. Immunol. 2012, 131, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Benedé, S.; López-Expósito, I.; Molina, E.; López-Fandiño, R. Egg proteins as allergens and the effects of the food matrix and processing. Food Funct. 2014, 6, 694–713. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Caubet, J.C.; Lin, J.; Ahrens, B.; Gimenez, G.; Bardina, L.; Niggemann, B.; Sampson, H.A.; Beyer, K. Natural tolerance development in cow’s milk allergic children: IgE and IgG4 epitope binding. Allergy 2017, 72, 1677–1685. [Google Scholar] [CrossRef]
- Kuitunen, M.; Englund, H.; Remes, S.; Movérare, R.; Pelkonen, A.; Borres, M.P.; Mäkelä, M.J. High IgE levels to α-lactalbumin, β-lactoglobulin and casein predict less successful cow’s milk oral immunotherapy. Allergy 2015, 70, 955–962. [Google Scholar] [CrossRef]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef]
- Yi, C.; Wambo, T.O. Factors affecting the interactions between beta-lactoglobulin and fatty acids as revealed in molecular dynamics simulations. Phys. Chem. Chem. Phys. 2015, 17, 23074–23080. [Google Scholar] [CrossRef] [Green Version]
- Fontana, A.; Spolaore, B.; De Laureto, P.P. The biological activities of protein/oleic acid complexes reside in the fatty acid. Biochim. Biophys. Acta Proteins Proteomics 2013, 1834, 1125–1143. [Google Scholar] [CrossRef]
- Gustafsson, L.; Leijonhufvud, I.; Aronsson, A.; Mossberg, A.-K.; Svanborg, C. Treatment of skin papillomas with topical α-lactalbumin–oleic acid. N. Engl. J. Med. 2004, 350, 2663–2672. [Google Scholar] [CrossRef] [Green Version]
- Mok, K.H.; Pettersson, J.; Orrenius, S.; Svanborg, C. HAMLET, protein folding, and tumor cell death. Biochem. Biophys. Res. Commun. 2007, 354, 1–7. [Google Scholar] [CrossRef]
- Brinkmann, C.R.; Heegaard, C.W.; Petersen, T.E.; Jensenius, J.C.; Thiel, S. The toxicity of bovine α-lactalbumin made lethal to tumor cells is highly dependent on oleic acid and induces killing in cancer cell lines and noncancer-derived primary cells. FEBS J. 2011, 278, 1955–1967. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Cvejić Hogervorst, J.H.; Garssen, J.; Wichers, H.J.; Willemsen, L.E.M. Long Chain Polyunsaturated Fatty Acids (LCPUFAs) in the Prevention of Food Allergy. Front. Immunol. 2019, 10, 1118. [Google Scholar] [CrossRef]
- Elsen, L.W.J.V.D.; van Esch, B.C.A.M.; Hofman, G.A.; Kant, J.; van de Heijning, B.J.M.; Garssen, J.; Willemsen, L.E.M. Dietary long chain n-3 polyunsaturated fatty acids prevent allergic sensitization to cow’s milk protein in mice. Clin. Exp. Allergy 2013, 43, 798–810. [Google Scholar] [CrossRef]
- Meng, X.; Zeng, Z.; Gao, J.; Tong, P.; Wu, Y.; Li, X.; Chen, H. Conformational changes in bovine alpha-lactalbumin and beta-lactoglobulin evoked by interaction with C18 unsaturated fatty acids provide insights into increased allergic potential. Food Funct. 2020, 11, 9240–9251. [Google Scholar] [CrossRef]
- Schägger, H.; Von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Foda, M.I.; Salama, H.H.; El-Sayed, M.; Hassan, Z.; Awad, R.; Otzen, D. Characteristic and cytotoxic activity of different a-Lactalbumin/fatty acids nanocomplex. Am. Int. J. Contemp. Sci. Res. 2015, 2, 148–162. [Google Scholar]
- Adel-Patient, K.; Wavrin, S.; Bernard, H.; Meziti, N.; Ah-Leung, S.; Wal, J.M. Oral tolerance and Treg cells are induced in BALB/c mice after gavage with bovine beta-lactoglobulin. Allergy 2011, 66, 1312–1321. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Sakaguchi, S. Molecular control of regulatory T cell development and function. Curr. Opin. Immunol. 2017, 49, 64–70. [Google Scholar] [CrossRef]
- Angelina, A.; Sirvent, S.; Palladino, C.; Vereda, A.; Cuesta-Herranz, J.; Eiwegger, T.; Rodríguez, R.; Breiteneder, H.; Villalba, M.; Palomares, O. The lipid-interaction capacity of Sin a 2 and Ara h 1, major mustard and peanut allergens of the cupin superfamily, endorses allergenicity. Allergy 2016, 71, 1284–1294. [Google Scholar] [CrossRef]
- Brinkmann, C.R.; Thiel, S.; Otzen, D.E. Protein-fatty acid complexes: Biochemistry, biophysics and function. FEBS J. 2013, 280, 1733–1749. [Google Scholar] [CrossRef]
- Nakamura, T.; Aizawa, T.; Kariya, R.; Okada, S.; Demura, M.; Kawano, K.; Makabe, K.; Kuwajima, K. Molecular Mechanisms of the Cytotoxicity of Human α-Lactalbumin Made Lethal to Tumor Cells (HAMLET) and Other Protein-Oleic Acid Complexes. J. Biol. Chem. 2013, 288, 14408–14416. [Google Scholar] [CrossRef] [Green Version]
- Kehoe, J.J.; Brodkorb, A. Interactions between sodium oleate and α-lactalbumin: The effect of temperature and concentration on complex formation. Food Hydrocoll. 2014, 34, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Hoppenbrouwers, T.; Fogliano, V.; Garssen, J.; Pellegrini, N.; Willemsen, L.E.M.; Wichers, H. Specific Polyunsaturated Fatty Acids Can Modulate in vitro Human moDC2s and Subsequent Th2 Cytokine Release. Front. Immunol. 2020, 11, 748. [Google Scholar] [CrossRef]
- Park, B.-K.; Park, S.; Park, J.-B.; Park, M.C.; Min, T.S.; Jin, M. Omega-3 fatty acids suppress Th2-associated cytokine gene expressions and GATA transcription factors in mast cells. J. Nutr. Biochem. 2013, 24, 868–876. [Google Scholar] [CrossRef]
- Kulka, M. Long-Chain n-3 Polyunsaturated Fatty Acids Inhibit Fcε Receptor I-Mediated Mast Cell Activation. J. Allergy Clin. Immunol. 2015, 135, AB64. [Google Scholar] [CrossRef]
- Nemashkalova, E.L.; Kazakov, A.S.; Khasanova, L.M.; Permyakov, E.A.; Permyakov, S.E. Structural Characterization of More Potent Alternatives to HAMLET, a Tumoricidal Complex of α-Lactalbumin and Oleic Acid. Biochemistry 2013, 52, 6286–6299. [Google Scholar] [CrossRef]
- Perez, A.A.; Andermatten, R.B.; Rubiolo, A.C.; Santiago, L.G. beta-Lactoglobulin heat-induced aggregates as carriers of polyunsaturated fatty acids. Food Chem. 2014, 158, 66–72. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Carrara, C.R.; Santiago, L.G. Complexes between ovalbumin nanoparticles and linoleic acid: Stoichiometric, kinetic and thermodynamic aspects. Food Chem. 2016, 211, 819–826. [Google Scholar] [CrossRef]
- Otzen, D. Protein-surfactant interactions: A tale of many states. Biochim. Biophys. Acta Proteins Proteomics 2011, 1814, 562–591. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Ferranti, P.; Fessas, D.; Nasi, A.; Rasmussen, P.; Iametti, S. Bound fatty acids modulate the sensitivity of bovine beta-lactoglobulin to chemical and physical denaturation. J. Agric. Food Chem. 2011, 59, 5729–5737. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Berin, M.C.; Arnaboldi, P.; Escalante, C.R.; Dahan, S.; Rauch, J.; Jensen-Jarolim, E.; Mayer, L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy 2008, 63, 882–890. [Google Scholar] [CrossRef]
- Del Moral, M.G.; Martínez-Naves, E. The Role of Lipids in Development of Allergic Responses. Immune Netw. 2017, 17, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Dearman, R.J.; Alcocer, M.J.C.; Kimber, I. Influence of plant lipids on immune responses in mice to the major Brazil nut allergen Ber e 1. Clin. Exp. Allergy 2007, 37, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.N.; Chatila, T.A. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Huang, S.; Yuan, X.; Liang, J.; Xu, R.; Yao, G.; Feng, X.; Sun, L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell. Mol. Immunol. 2015, 14, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Saidova, A.; Hershkop, A.M.; Ponce, M.; Eiwegger, T. Allergen-Specific T Cells in IgE-Mediated Food Allergy. Arch. Immunol. Ther. Exp. 2017, 66, 161–170. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Liu, J.; Cao, X. Cellular and molecular regulation of innate inflammatory responses. Cell. Mol. Immunol. 2016, 13, 711–721. [Google Scholar] [CrossRef]
- Shik, D.; Tomar, S.; Lee, J.-B.; Chen, C.-Y.; Smith, A.; Wang, Y.-H. IL-9-producing cells in the development of IgE-mediated food allergy. Semin. Immunopathol. 2016, 39, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-Y.; Lee, J.-B.; Liu, B.; Ohta, S.; Wang, P.-Y.; Kartashov, A.V.; Mugge, L.; Abonia, J.P.; Barski, A.; Izuhara, K.; et al. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity 2015, 43, 788–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Wu, Y.; Wen, X.; Gao, J.; Xie, Y.; Zhao, X.; Yuan, J.; Yang, H.; Zeng, Z.; Li, X.; et al. Dietary Linolenic Acid Increases Sensitizing and Eliciting Capacities of Cow’s Milk Whey Proteins in BALB/c Mice. Nutrients 2022, 14, 822. https://doi.org/10.3390/nu14040822
Meng X, Wu Y, Wen X, Gao J, Xie Y, Zhao X, Yuan J, Yang H, Zeng Z, Li X, et al. Dietary Linolenic Acid Increases Sensitizing and Eliciting Capacities of Cow’s Milk Whey Proteins in BALB/c Mice. Nutrients. 2022; 14(4):822. https://doi.org/10.3390/nu14040822
Chicago/Turabian StyleMeng, Xuanyi, Yong Wu, Xuefang Wen, Jinyan Gao, Yanhai Xie, Xiaoli Zhao, Jin Yuan, Hao Yang, Zheling Zeng, Xin Li, and et al. 2022. "Dietary Linolenic Acid Increases Sensitizing and Eliciting Capacities of Cow’s Milk Whey Proteins in BALB/c Mice" Nutrients 14, no. 4: 822. https://doi.org/10.3390/nu14040822
APA StyleMeng, X., Wu, Y., Wen, X., Gao, J., Xie, Y., Zhao, X., Yuan, J., Yang, H., Zeng, Z., Li, X., & Chen, H. (2022). Dietary Linolenic Acid Increases Sensitizing and Eliciting Capacities of Cow’s Milk Whey Proteins in BALB/c Mice. Nutrients, 14(4), 822. https://doi.org/10.3390/nu14040822