Vitamin D Levels in Early and Middle Pregnancy and Preeclampsia, a Systematic Review and Meta-Analysis
Abstract
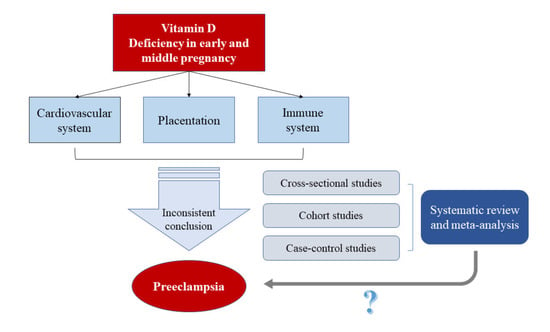
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Selection Process
2.4. Risk of Bias Assessment
2.5. Data Collection Process
2.6. Synthesis Methods
3. Results
3.1. Characteristics of the Included Studies
3.2. Replete Levels of VitD (≥30 ng/mL) versus Insufficient or Deficient Levels of VitD (<30 ng/mL)
3.3. Replete or Insufficient Levels of VitD (≥20 ng/mL) versus Deficient Levels of VitD (<20 ng/mL or < 15 ng/mL)
3.4. Replete Levels of VitD (≥30 ng/mL) versus Insufficient Levels of VitD (20–30 ng/mL or 15–30 ng/mL)
3.5. Replete Levels of VitD (≥30 ng/mL) versus Deficient Levels of VitD (<20 ng/mL or <15 ng/mL)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Macedo, T.C.C.; Montagna, E.; Trevisan, C.M.; Zaia, V.; de Oliveira, R.; Barbosa, C.P.; Laganà, A.S.; Bianco, B. Prevalence of preeclampsia and eclampsia in adolescent pregnancy: A systematic review and meta-analysis of 291,247 adolescents worldwide since 1969. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 177–186. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Malinowski, A.; Zhou, Q.; Yusuf, S.; Devereaux, P.J. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am. Heart J. 2008, 156, 918–930. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Han, Z.; Walsh, M.W.; Gerstein, H.C.; Devereaux, P.J. Kidney disease after preeclampsia: A systematic review and meta-analysis. Am. J. Kidney Dis. 2010, 55, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Covella, B.; Vinturache, A.E.; Cabiddu, G.; Attini, R.; Gesualdo, L.; Versino, E.; Piccoli, G.B. A systematic review and meta-analysis indicates long-term risk of chronic and end-stage kidney disease after preeclampsia. Kidney Int. 2019, 96, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.K.; Crozier, S.R.; Harvey, N.C.; Gale, C.R.; Dennison, E.M.; Boucher, B.J.; Arden, N.K.; Godfrey, K.M.; Cooper, C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: A longitudinal study. Lancet 2006, 367, 36–43. [Google Scholar] [CrossRef]
- Karras, S.N.; Anagnostis, P.; Annweiler, C.; Naughton, D.P.; Petroczi, A.; Bili, E.; Harizopoulou, V.; Tarlatzis, B.C.; Persinaki, A.; Papadopoulou, F.; et al. Maternal vitamin D status during pregnancy: The Mediterranean reality. Eur. J. Clin. Nutr. 2014, 68, 864–869. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo. Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Kiely, M.E.; Wagner, C.L.; Roth, D.E. Vitamin D in pregnancy: Where we are and where we should go. J. Steroid. Biochem. Mol. Biol. 2020, 201, 105669. [Google Scholar] [CrossRef]
- Wetta, L.A.; Biggio, J.R.; Cliver, S.; Abramovici, A.; Barnes, S.; Tita, A.T. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? Am. J. Perinatol. 2014, 31, 541–546. [Google Scholar] [PubMed] [Green Version]
- Chan, S.Y.; Susarla, R.; Canovas, D.; Vasilopoulou, E.; Ohizua, O.; McCabe, C.J.; Hewison, M.; Kilby, M.D. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta 2015, 36, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poniedziałek-Czajkowska, E.; Mierzyński, R. Could Vitamin D Be Effective in Prevention of Preeclampsia? Nutrients 2021, 13, 3854. [Google Scholar] [CrossRef] [PubMed]
- Schröder-Heurich, B.; von Hardenberg, S.; Brodowski, L.; Kipke, B.; Meyer, N.; Borns, K.; von Kaisenberg, C.S.; Brinkmann, H.; Claus, P.; von Versen-Höynck, F. Vitamin D improves endothelial barrier integrity and counteracts inflammatory effects on endothelial progenitor cells. Faseb. J. 2019, 33, 9142–9153. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, L.; Burlakov, J.; Myerski, A.C.; von Kaisenberg, C.S.; Grundmann, M.; Hubel, C.A.; von Versen-Höynck, F. Vitamin D prevents endothelial progenitor cell dysfunction induced by sera from women with preeclampsia or conditioned media from hypoxic placenta. PLoS ONE 2014, 9, e98527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef]
- Behjat Sasan, S.; Zandvakili, F.; Soufizadeh, N.; Baybordi, E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017, 2017, 8249264. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.N.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Vitamin D and placental-decidual function. J. Soc. Gynecol. Investig. 2004, 11, 263–271. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Scaletti, C.; Maggi, E.; Romagnani, S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J. Neuroimmunol. 2000, 109, 30–33. [Google Scholar] [CrossRef]
- Achkar, M.; Dodds, L.; Giguère, Y.; Forest, J.C.; Armson, B.A.; Woolcott, C.; Agellon, S.; Spencer, A.; Weiler, H.A. Vitamin D status in early pregnancy and risk of preeclampsia. Am. J. Obstet. Gynecol. 2015, 212, e511–e517. [Google Scholar] [CrossRef] [PubMed]
- Baca, K.M.; Simhan, H.N.; Platt, R.W.; Bodnar, L.M. Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann. Epidemiol. 2016, 26, 853–857.e1. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.C.; Guío, E.; Quintero-Lesmes, D.C.; Becerra-Bayona, S.; Luna-Gonzalez, M.L.; Herrera, V.M.; Prada, C.E. Vitamin D deficiency and pre-eclampsia in Colombia: PREVitD study. Pregnancy Hypertens 2018, 14, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O’Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. 2016, 126, 4702–4715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powe, C.E.; Seely, E.W.; Rana, S.; Bhan, I.; Ecker, J.; Karumanchi, S.A.; Thadhani, R. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension 2010, 56, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Su, L.; Liu, M.; Liu, Y.; Cao, X.; Wang, Z.; Xiao, H. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: A prospective observational study in southern China. Eur. J. Clin. Nutr. 2014, 68, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Cordero, M.J.; Lasserrot-Cuadrado, A.; Mur-Villar, N.; León-Ríos, X.A.; Rivero-Blanco, T.; Pérez-Castillo, I.M. Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies. Midwifery 2020, 87, 102707. [Google Scholar] [CrossRef]
- Akbari, S.; Khodadadi, B.; Ahmadi, S.A.Y.; Abbaszadeh, S.; Shahsavar, F. Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: A systematic review and updated meta-analysis. Taiwan J. Obstet. Gynecol. 2018, 57, 241–247. [Google Scholar] [CrossRef]
- Serrano-Díaz, N.C.; Gamboa-Delgado, E.M.; Domínguez-Urrego, C.L.; Vesga-Varela, A.L.; Serrano-Gómez, S.E.; Quintero-Lesmes, D.C. Vitamin D and risk of preeclampsia: A systematic review and meta-analysis. Biomedica 2018, 38 (Suppl. S1), 43–53. [Google Scholar] [CrossRef]
- Wei, S.Q.; Qi, H.P.; Luo, Z.C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Fetal. Neonatal. Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. In Proceedings of the 3rd Symposium on Systematic Reviews: Beyond the Basics. Improving Quality and Impact, Oxford, UK, 3–5 July 2000; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 July 2000).
- Baker, A.M.; Haeri, S.; Camargo, C.A., Jr.; Espinola, J.A.; Stuebe, A.M. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J. Clin. Endocrinol. Metab. 2010, 95, 5105–5109. [Google Scholar] [CrossRef] [PubMed]
- Schneuer, F.J.; Roberts, C.L.; Guilbert, C.; Simpson, J.M.; Algert, C.S.; Khambalia, A.Z.; Tasevski, V.; Ashton, A.W.; Morris, J.M.; Nassar, N. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am. J. Clin. Nutr. 2014, 99, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benachi, A.; Baptiste, A.; Taieb, J.; Tsatsaris, V.; Guibourdenche, J.; Senat, M.V.; Haidar, H.; Jani, J.; Guizani, M.; Jouannic, J.M.; et al. Relationship between vitamin D status in pregnancy and the risk for preeclampsia: A nested case-control study. Clin. Nutr. 2020, 39, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alonso, A.M.; Dionis-Sánchez, E.C.; Chedraui, P.; González-Salmerón, M.D.; Pérez-López, F.R. First-trimester maternal serum 25-hydroxyvitamin D₃ status and pregnancy outcome. Int. J. Gynaecol. Obstet. 2012, 116, 6–9. [Google Scholar] [CrossRef]
- Christoph, P.; Challande, P.; Raio, L.; Surbek, D. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss. Med. Wkly. 2020, 150, w20238. [Google Scholar] [CrossRef]
- Al-Shaikh, G.K.; Ibrahim, G.H.; Fayed, A.A.; Al-Mandeel, H. Impact of vitamin D deficiency on maternal and birth outcomes in the Saudi population: A cross-sectional study. BMC Pregnancy Childbirth 2016, 16, 119. [Google Scholar] [CrossRef] [Green Version]
- Hemmingway, A.; Kenny, L.C.; Malvisi, L.; Kiely, M.E. Exploring the concept of functional vitamin D deficiency in pregnancy: Impact of the interaction between 25-hydroxyvitamin D and parathyroid hormone on perinatal outcomes. Am. J. Clin. Nutr. 2018, 108, 821–829. [Google Scholar] [CrossRef]
- Wei, S.Q.; Audibert, F.; Hidiroglou, N.; Sarafin, K.; Julien, P.; Wu, Y.; Luo, Z.C.; Fraser, W.D. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. Bjog 2012, 119, 832–839. [Google Scholar] [CrossRef]
- Flood-Nichols, S.K.; Tinnemore, D.; Huang, R.R.; Napolitano, P.G.; Ippolito, D.L. Vitamin D deficiency in early pregnancy. PLoS ONE 2015, 10, e0123763. [Google Scholar] [CrossRef] [PubMed]
- Shand, A.W.; Nassar, N.; Von Dadelszen, P.; Innis, S.M.; Green, T.J. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. Bjog 2010, 117, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- van Weert, B.; van den Berg, D.; Hrudey, E.J.; Oostvogels, A.; de Miranda, E.; Vrijkotte, T.G.M. Is first trimester vitamin D status in nulliparous women associated with pregnancy related hypertensive disorders? Midwifery 2016, 34, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnus, M.C.; Miliku, K.; Bauer, A.; Engel, S.M.; Felix, J.F.; Jaddoe, V.W.V.; Lawlor, D.A.; London, S.J.; Magnus, P.; McGinnis, R.; et al. Vitamin D and risk of pregnancy related hypertensive disorders: Mendelian randomisation study. BMJ 2018, 361, k2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, T.O.; Chen, X.; Stein, T.P. Vitamin D, secondary hyperparathyroidism, and preeclampsia. Am. J. Clin. Nutr. 2013, 98, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Fernández, I.; Prieto, B.; Rodríguez, V.; Ruano, Y.; Escudero, A.I.; Álvarez, F.V. Role of vitamin D and sFlt-1/PlGF ratio in the development of early- and late-onset preeclampsia. Clin. Chem. Lab. Med. 2015, 53, 1033–1040. [Google Scholar] [CrossRef]
- Gidlöf, S.; Silva, A.T.; Gustafsson, S.; Lindqvist, P.G. Vitamin D and the risk of preeclampsia--a nested case-control study. Acta Obstet. Gynecol. Scand. 2015, 94, 904–908. [Google Scholar] [CrossRef]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, N.; Matsuo, H.; Murakoshi, H.; Laoag-Fernandez, J.B.; Samoto, T.; Maruo, T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002, 186, 158–166. [Google Scholar] [CrossRef]
- Perez-Sepulveda, A.; Torres, M.J.; Khoury, M.; Illanes, S.E. Innate immune system and preeclampsia. Front. Immunol. 2014, 5, 244. [Google Scholar] [CrossRef] [Green Version]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaños-Miranda, A.; Navarro-Romero, C.S.; Sillas-Pardo, L.J.; Ramírez-Valenzuela, K.L.; Isordia-Salas, I.; Jiménez-Trejo, L.M. Soluble Endoglin As a Marker for Preeclampsia, Its Severity, and the Occurrence of Adverse Outcomes. Hypertension 2019, 74, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Borzychowski, A.M.; Sargent, I.L.; Redman, C.W. Inflammation and pre-eclampsia. Semin. Fetal Neonatal Med. 2006, 11, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P.; Deloia, J.A. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin. Immunol. 2006, 118, 268–275. [Google Scholar] [CrossRef]
- Hyppönen, E.; Cavadino, A.; Williams, D.; Fraser, A.; Vereczkey, A.; Fraser, W.D.; Bánhidy, F.; Lawlor, D.; Czeizel, A.E. Vitamin D and Pre-Eclampsia: Original Data, Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2013, 63, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Tabesh, M.; Salehi-Abargouei, A.; Tabesh, M.; Esmaillzadeh, A. Maternal vitamin D status and risk of pre-eclampsia: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 3165–3173. [Google Scholar] [CrossRef] [Green Version]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.H.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F.G. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020, 39, 1742–1752. [Google Scholar] [CrossRef]
- Fu, Z.M.; Ma, Z.Z.; Liu, G.J.; Wang, L.L.; Guo, Y. Vitamins supplementation affects the onset of preeclampsia. J. Formos. Med. Assoc. 2018, 117, 6–13. [Google Scholar] [CrossRef]
- Khaing, W.; Vallibhakara, S.A.; Tantrakul, V.; Vallibhakara, O.; Rattanasiri, S.; McEvoy, M.; Attia, J.; Thakkinstian, A. Calcium and Vitamin D Supplementation for Prevention of Preeclampsia: A Systematic Review and Network Meta-Analysis. Nutrients 2017, 9, 1141. [Google Scholar] [CrossRef] [Green Version]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef] [Green Version]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, Cd008873. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Pasupuleti, V.; Mezones-Holguin, E.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Hernandez, A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2015, 103, 1278–1288.e4. [Google Scholar] [CrossRef] [PubMed]




| Studies | Study Type | Eligibility for Pregnant Women | Method of Measurement | Gestational Weeks of Sampling | Sample Size | Location |
|---|---|---|---|---|---|---|
| [11] | Nested case–control | Women with multiple gestations, fetal anomalies, or maternal medical complications were excluded. | LCMS | 15–21 | 266 | Birmingham, America/52.3° N |
| [33] | Nested case–control | Women with multiple gestations, major congenital fetal anomalies, pregestational hypertension, kidney disease, diabetes mellitus, known thrombophilias, PCMD were excluded. | LCMS | 15–20 | 241 | Boston, America /52.58° N |
| [26] | Cohort | Women with abnormal liver function, chronic disease and tumor; severe infections, trauma or in perioperative, before 13 weeks of gestation, and women who take corticosteroids, drug abuse (including alcohol) were excluded. | ECLIA | 16–20 | 1953 | Guangzhou, China/23.1° N |
| [34] | Nested case–control | Women with adverse pregnancy outcomes were excluded | CLIA | 10–14 | 5109 | New South Wales, Australia /33.9° |
| [25] | Nested case–control | Women with GDM or give birth to SGA infants | LCMS | ≤14 | 170 | Boston, America /52.58° N |
| [22] | Nested case–control | Women who had aneuploidy screening at 20 weeks or less gestation and who subsequently delivered live born infants. | LCMS | ≤20 | 2327 | Pennsylvania, America/40.3° |
| [35] | Nested case–control | Nulliparous women aged 14–44 years, carrying singleton infants. | ELISA | ≤22 | 265 | Pennsylvania, America/40.3° |
| [36] | Nested case–control | Women with multiple gestations, calcium imbalance, hypertension, renal insufficiency, bone disease, lithium therapy, bowel malabsorption, or kidney stone disease were excluded. | RIA | ≤15 | 402 | six centers: one in Belgium and five in France |
| [21] | Nested case–control | Women with preexisting hypertension, missing essential outcome information (no gestational age at enrollment), or multiple gestations were excluded. | CLIA | ≤20 | 2048 | Quebec, Canada/46.5° N |
| [37] | Cross-sectional | Women with increased risks for intrauterine fetal growth restriction, hereditary thrombophilias, or acquired thrombophilias were excluded. | ECLIA | 11–14 | 466 | Almería, Spain/36.8° |
| [38] | Cross-sectional | NA | CLIA | ≤24 | 1382 | Bern, Switzerland/46.5° |
| [39] | Cross-sectional | Women with PCMD, metabolic bone disease, liver, kidney, or gastrointestinal diseases and the use of vitamin D supplements. | ELISA | ≤12 | 1000 | Saudi Arabia/24.3° |
| [40] | Cohort | Nulliparous women with a low-risk singleton pregnancy. Pregnancies at increased risk of pre-eclampsia, SGA, or spontaneous preterm birth or medical history, known major fetal anomaly or abnormal karyotype were excluded. | LCMS | <16 | 1754 | Cork, Ireland/51.9° N |
| [24] | Nested case–control | Maternal age between 18 and 39 years and not a current smoker or a user of other nicotine products. Women with PCMD, multiple pregnancies, vitamin D taken (>2000 IU per day), fetal anomalies, or ART use were excluded. | CLIA | 10–18 | 157 | United States |
| [41] | Cohort | Women who regularly took 200 mg/d for vitamin C and/or 50 IU/d for vitamin E, or warfarin, or with fetal abnormalities, or with PCMD, or with repeated spontaneous abortion were excluded. | CLIA | 12–18 | 697 | Canada and Mexico |
| [42] | Cohort | Healthy, nulliparous women aged 18 years or older without PCMD or infertility treatment. Patients with predictors for hypovitaminosis D or a prior pregnancy that had progressed beyond the first trimester and resulted in a fetal loss were excluded. | ELISA | 8–12 | 235 | United States |
| [43] | Cohort | Women aged ≥ 18 years with either clinical or biochemical risk factors for pre-eclampsia | RIA | 10–20 | 221 | Canada/49° N |
| [44] | Cohort | Nulliparous women with a singleton pregnancy. | ELISA | <17 | 2074 | Amsterdam, the Netherlands |
| [45] | Cohort | Gestational age < 24 weeks, resident in Rotterdam at the date of delivery, expected delivery date lies between June 2002 and July 2004 | LCMS | <24 | 3323 | Rotterdam, the Netherlands |
| [46] | Cohort | Healthy pregnant women. Gravidae with serious nonobstetric problems are not eligible. | LCMS | <20 | 1141 | Camden, United States |
| [47] | Nested case–control | Suspected PE over 20 weeks of gestation between January 2010 and March 2013. Women who were diagnosed with PE before their presentation at the emergency department were not included. | CLIA | 9–12 | 142 | Oviedo, Spain |
| [48] | Nested case–control | After identifying women who developed preeclampsia, the control group was drawn by random selection and comprised 10 women delivered in each month of the year | CLIA | Mean (SD): 12 (3) | 157 | Malmo, Sweden /55°37′ N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.-L.; Zhang, C.-X.; Chen, P.; Zhang, D.; Hunt, S. Vitamin D Levels in Early and Middle Pregnancy and Preeclampsia, a Systematic Review and Meta-Analysis. Nutrients 2022, 14, 999. https://doi.org/10.3390/nu14050999
Hu K-L, Zhang C-X, Chen P, Zhang D, Hunt S. Vitamin D Levels in Early and Middle Pregnancy and Preeclampsia, a Systematic Review and Meta-Analysis. Nutrients. 2022; 14(5):999. https://doi.org/10.3390/nu14050999
Chicago/Turabian StyleHu, Kai-Lun, Chun-Xi Zhang, Panpan Chen, Dan Zhang, and Sarah Hunt. 2022. "Vitamin D Levels in Early and Middle Pregnancy and Preeclampsia, a Systematic Review and Meta-Analysis" Nutrients 14, no. 5: 999. https://doi.org/10.3390/nu14050999
APA StyleHu, K. -L., Zhang, C. -X., Chen, P., Zhang, D., & Hunt, S. (2022). Vitamin D Levels in Early and Middle Pregnancy and Preeclampsia, a Systematic Review and Meta-Analysis. Nutrients, 14(5), 999. https://doi.org/10.3390/nu14050999