High Compliance to Mediterranean Diet Associates with Lower Platelet Activation and Liver Collagen Deposition in Patients with Nonalcoholic Fatty Liver Disease
Abstract
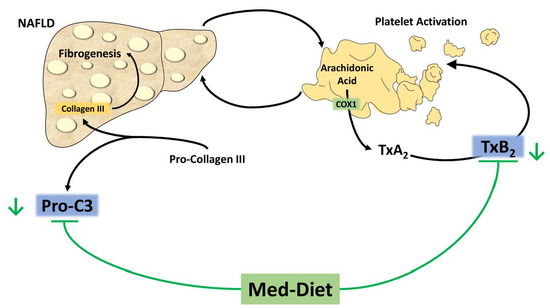
:1. Introduction
2. Materials and Methods
2.1. Med-Diet Questionnaire
2.2. Serum TxB2 Assay
2.3. Pro-C3 Assay
2.4. Statistical Analysis
3. Results
3.1. Patients’ Charachteristics According to Med-Diet Adherence
3.2. Med-Diet Score and TxB2
3.3. Med-Diet Score and Pro-C3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNESCO. Representative List of the Intangible Culture Heritage of Humanity. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 22 January 2022).
- Gussow, J.D. Mediterranean diets: Are they environmentally responsible? Am. J. Clin. Nutr. 1995, 61, 1383S–1389S. [Google Scholar] [CrossRef] [PubMed]
- Pairotti, M.B.; Cerutti, A.K.; Martini, F.; Vesce, E.; Padovan, D.; Beltramo, R. Energy consumption and GHG emission of the Mediterranean diet: A systemic assessment using a hybrid LCA-IO method. J. Clean. Prod. 2015, 103, 507–516. [Google Scholar] [CrossRef]
- Boucher, J.L. Mediterranean Eating Pattern. Diabetes Spectr. 2017, 30, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keys, A.; Aravanis, C.; Blackburn, H.W.; Van Buchem, F.S.; Buzina, R.; Djordjević, B.D.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. Epidemiological studies related to coronary heart disease: Characteristics of men aged 40–59 in seven countries. Acta Med. Scand. Suppl. 1966, 460, 1–392. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharm. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Ben, M.; Polimeni, L.; Brancorsini, M.; Di Costanzo, A.; D’Erasmo, L.; Baratta, F.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Violi, F.; et al. Non-alcoholic fatty liver disease, metabolic syndrome and patatin-like phospholipase domain-containing protein3 gene variants. Eur. J. Intern. Med. 2014, 25, 566–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EASL-EASD-EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Boutari, C.; Chrysohoou, C.; Fragkopoulou, E.; Antonopoulou, S.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B.; Mantzoros, C.S.; Investigators, A.s. Mediterranean diet is inversely associated with steatosis and fibrosis and decreases ten-year diabetes and cardiovascular risk in NAFLD subjects: Results from the ATTICA prospective cohort study. Clin. Nutr. 2021, 40, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.; Tiniakos, D.; Schattenberg, J.M.; Ratziu, V.; Bugianessi, E.; Petta, S.; Oliveira, C.P.; Govaere, O.; Younes, R.; McPherson, S.; et al. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Rep. 2019, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar] [PubMed]
- Gressner, A.M.; Weiskirchen, R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J. Cell. Mol. Med. 2006, 10, 76–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, A.; Zimmermann, A.; Weigand, K. The N-terminal propeptide of collagen type III in serum reflects activity and degree of fibrosis in patients with chronic liver disease. Hepatology 1984, 4, 830–834. [Google Scholar] [CrossRef]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef]
- Baratta, F.; Pastori, D.; Bartimoccia, S.; Cammisotto, V.; Cocomello, N.; Colantoni, A.; Nocella, C.; Carnevale, R.; Ferro, D.; Angelico, F.; et al. Poor Adherence to Mediterranean Diet and Serum Lipopolysaccharide are Associated with Oxidative Stress in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Charlton, M.; Kawaguchi, A.; Yamamura, S.; Nakano, D.; Tsutsumi, T.; Zafer, M.; Torimura, T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin. Liver Dis. 2021, 41, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased liver localization of lipopolysaccharides in human and experimental non-alcoholic fatty liver disease. Hepatology 2019, 72, 470–485. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef] [Green Version]
- Hernáez, Á.; Castañer, O.; Tresserra-Rimbau, A.; Pintó, X.; Fitó, M.; Casas, R.; Martínez-González, M.; Corella, D.; Salas-Salvadó, J.; Lapetra, J.; et al. Mediterranean Diet and Atherothrombosis Biomarkers: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2020, 64, 2000350. [Google Scholar] [CrossRef]
- Pignatelli, P.; Pastori, D.; Farcomeni, A.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Bucci, T.; Carnevale, R.; Violi, F. Mediterranean diet reduces thromboxane A2 production in atrial fibrillation patients. Clin. Nutr. 2015, 34, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Fernández-Jarne, E.; Serrano-Martínez, M.; Wright, M.; Gomez-Gracia, E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur. J. Clin. Nutr. 2004, 58, 1550–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureda, A.; Bibiloni, M.D.; Martorell, M.; Buil-Cosiales, P.; Marti, A.; Pons, A.; Tur, J.A.; Martinez-Gonzalez, M.; Investigators, P.S. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: The PREDIMED study. Mol. Nutr. Food Res. 2016, 60, 2654–2664. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Pastori, D.; Baratta, F.; Novo, M.; Cocomello, N.; Violi, F.; Angelico, F.; Del Ben, M. Remnant Lipoprotein Cholesterol and Cardiovascular and Cerebrovascular Events in Patients with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2018, 7, 378. [Google Scholar] [CrossRef] [Green Version]
- Abeles, R.D.; Mullish, B.H.; Forlano, R.; Kimhofer, T.; Adler, M.; Tzallas, A.; Giannakeas, N.; Yee, M.; Mayet, J.; Goldin, R.D.; et al. Derivation and validation of a cardiovascular risk score for prediction of major acute cardiovascular events in non-alcoholic fatty liver disease; the importance of an elevated mean platelet volume. Aliment. Pharm. 2019, 49, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Pastori, D.; Loffredo, L.; Perri, L.; Baratta, F.; Scardella, L.; Polimeni, L.; Pani, A.; Brancorsini, M.; Albanese, F.; Catasca, E.; et al. Relation of nonalcoholic fatty liver disease and Framingham Risk Score to flow-mediated dilation in patients with cardiometabolic risk factors. Am. J. Cardiol. 2015, 115, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lee, G.; Heo, S.Y.; Roh, Y.S. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef]
- Renga, B.; Scavizzi, F. Platelets and cardiovascular risk. Acta Cardiol. 2017, 72, 2–8. [Google Scholar] [CrossRef]
- FitzGerald, G.A. Mechanisms of platelet activation: Thromboxane A2 as an amplifying signal for other agonists. Am. J. Cardiol. 1991, 68, 11B–15B. [Google Scholar] [CrossRef]
- Catella, F.; Healy, D.; Lawson, J.A.; FitzGerald, G.A. 11-Dehydrothromboxane B2: A quantitative index of thromboxane A2 formation in the human circulation. Proc. Natl. Acad. Sci. USA 1986, 83, 5861–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastori, D.; Pignatelli, P.; Farcomeni, A.; Nocella, C.; Bartimoccia, S.; Carnevale, R.; Violi, F. Age-related increase of thromboxane B2 and risk of cardiovascular disease in atrial fibrillation. Oncotarget 2016, 7, 39143–39147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cangemi, R.; Casciaro, M.; Rossi, E.; Calvieri, C.; Bucci, T.; Calabrese, C.M.; Taliani, G.; Falcone, M.; Palange, P.; Bertazzoni, G.; et al. Platelet activation is associated with myocardial infarction in patients with pneumonia. J. Am. Coll. Cardiol. 2014, 64, 1917–1925. [Google Scholar] [CrossRef] [Green Version]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Umair Ijaz, M.; Hussain, M.; Ali Khan, I.; Mehmood, N.; Siddiqi, S.M.; Liu, C.; Zhao, D.; Xu, X.; Zhou, G.; et al. High fat diet incorporated with meat proteins changes biomarkers of lipid metabolism, antioxidant activities, and the serum metabolomic profile in Glrx1. Food Funct. 2020, 11, 236–252. [Google Scholar] [CrossRef]
- Carnevale, R.; Pastori, D.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Novo, M.; Del Ben, M.; Farcomeni, A.; Angelico, F.; Violi, F. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: Effect of extra-virgin olive oil. Eur. J. Nutr. 2019, 58, 843–851. [Google Scholar] [CrossRef]
- Violi, F.; Loffredo, L.; Carnevale, R.; Pignatelli, P.; Pastori, D. Atherothrombosis and Oxidative Stress: Mechanisms and Management in Elderly. Antioxid. Redox Signal. 2017, 27, 1083–1124. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Galeone, C.; Negri, E.; La Vecchia, C. Trends in adherence to the Mediterranean diet in an Italian population between 1991 and 2006. Eur. J. Clin. Nutr. 2010, 64, 1052–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allman-Farinelli, M.; Partridge, S.R.; Roy, R. Weight-Related Dietary Behaviors in Young Adults. Curr. Obes. Rep. 2016, 5, 23–29. [Google Scholar] [CrossRef]
- Šarac, J.; Havaš Auguštin, D.; Lovrić, M.; Stryeck, S.; Šunić, I.; Novokmet, N.; Missoni, S. A Generation Shift in Mediterranean Diet Adherence and Its Association with Biological Markers and Health in Dalmatia, Croatia. Nutrients 2021, 13, 4564. [Google Scholar] [CrossRef]
- García Cabrera, S.; Herrera Fernández, N.; Rodríguez Hernández, C.; Nissensohn, M.; Román-Viñas, B.; Serra-Majem, L. KIDMED test; Prevalence of low adherence to the Mediterranean diet in children and young.; A systematic review. Nutr. Hosp. 2015, 32, 2390–2399. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Marin-Alejandre, B.A.; Abete, I.; Cantero, I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Quiroga, J.; Martinez-Echeverria, A.; Uriz-Otano, J.I.; et al. The Metabolic and Hepatic Impact of Two Personalized Dietary Strategies in Subjects with Obesity and Nonalcoholic Fatty Liver Disease: The Fatty Liver in Obesity (FLiO) Randomized Controlled Trial. Nutrients 2019, 11, 2543. [Google Scholar] [CrossRef] [Green Version]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef] [PubMed]

| Adherence to Mediterranean Diet | |||||
|---|---|---|---|---|---|
| Low (Score 0–2 pts) (n = 67) | Intermediate (Score 3–6 pts) (n = 487) | High (Score 7–9 pts) (n = 101) | pamong all | plow vs. high | |
| Age (years) | 51.3 ± 13.1 | 55.3 ± 11.4 | 55.2 ± 11.6 | 0.028 | 0.092 |
| Female (%) | 34.3 | 40.7 | 31.7 | 0.181 | 0.721 |
| BMI (Kg/m2) | 31.1 ± 5.4 | 30.5 ± 5.0 | 29.7 ± 4.3 | 0.144 | 0.185 |
| Obesity (BMI ≥ 30 Kg/m2) (%) | 53.7 | 49.3 | 41.6 | 0.248 | 0.122 |
| Metabolic syndrome (%) | 70.1 | 61.1 | 52.5 | 0.063 | 0.022 |
| Waist circumference (cm) | 108.2 ± 12.8 | 107.2 ± 12.0 | 105.8 ± 9.9 | 0.392 | 0.581 |
| Diabetes (%) | 25.4 | 29.2 | 27.7 | 0.797 | 0.736 |
| Glycaemia (mg/dL) | 107.0 ± 38.4 | 106.1 ± 27.7 | 102.9 ± 19.4 | 0.531 | 1.000 |
| Antiplatelet drugs (%) | 20.9 | 15.6 | 11.9 | 0.288 | 0.114 |
| Statin use (%) | 40.3 | 39.0 | 33.7 | 0.566 | 0.381 |
| Arterial hypertension (%) | 56.7 | 61.4 | 55.4 | 0.456 | 0.871 |
| Total cholesterol (mg/dL) | 202.1 ± 43.2 | 196.5 ± 42.1 | 195.5 ± 38.7 | 0.554 | 0.950 |
| HDL (mg/dL) | 46.4 ± 10.7 | 47.9 ± 14.1 | 47.5 ± 12.7 | 0.702 | 1.000 |
| Total cholesterol/HDL | 4.6 ± 1.4 | 4.4 ± 1.7 | 4.4 ± 1.3 | 0.690 | 1.000 |
| Triglycerides (mg/dL) | 164.0 (114.0–212.0) | 137.0 (103.0–183.0) | 127.0 (103.0–163.0) | 0.026 | 0.007 |
| GGT (UI/L) | 30.5 (19.7–44.2) | 28.0 (17.0–42.0) | 23.0 (17.0–33.2) | 0.074 | 0.028 |
| AST (UI/L) | 21.5 (18.0–28.2) | 22.0 (18.0–29.0) | 20.0 (17.0–26.7) | 0.225 | 0.265 |
| ALT (UI/L) | 32.0 (20.0–43.0) | 28.0 (20.0–44.0) | 25.0 (18.0–35.0) | 0.093 | 0.054 |
| Platelets | 250.9 ± 60.4 | 237.6 ± 63.0 | 230.4 ± 52.3 | 0.109 | 0.108 |
| AST-to-Platelet ratio | 0.3 (0.2–0.3) | 0.3 (0.2–0.4) | 0.3 (0.2–0.3) | 0.465 | 0.900 |
| TxB2 (pg/mL) | 191.4 ± 32.6 | 185.4 ± 33.2 | 177.5 ± 24.1 | 0.015 | 0.017 |
| Pro-C3 (ng/mL) | 7.8 ± 3.0 | 7.3 ± 2.7 | 6.3 ± 1.7 | <0.001 | <0.001 |
| Panel A | B | S.E. | Beta | p |
|---|---|---|---|---|
| Age | −0.26 | 0.11 | −0.09 | 0.022 |
| Female sex | 2.73 | 2.64 | 0.04 | 0.300 |
| Total-c/HDL-c | 0.58 | 1.19 | 0.02 | 0.625 |
| Glycaemia | 0.02 | 0.05 | 0.02 | 0.661 |
| BMI | −0.06 | 0.25 | −0.01 | 0.809 |
| Med-Diet score | −1.93 | 0.74 | −0.10 | 0.009 |
| Triglycerides * | −8.19 | 7.53 | −0.05 | 0.277 |
| APRI * | 35.38 | 5.47 | 0.25 | <0.001 |
| Panel A | III TxB2 Tertile * | II TxB2 Tertile * | ||
|---|---|---|---|---|
| Odds Ratio (95% C.I for OR) | p | Odds Ratio (95% C.I for OR) | p | |
| Age ≥ 65 years | 0.99 (0.62–1.57) | 0.956 | 0.50 (0.30–0.85) | 0.010 |
| Female Sex | 1.20 (0.81–1.78) | 0.364 | 1.42 (0.95–2.13) | 0.087 |
| Metabolic syndrome | 0.92 (0.62–1.36) | 0.682 | 1.34 (0.89–2.01) | 0.166 |
| Antiplatelet therapy | 0.91 (0.52–1.59) | 0.734 | 1.44 (0.84–2.47) | 0.182 |
| APRI > 0.7 | 2.39 (1.05–5.47) | 0.039 | 1.13 (0.42–3.06) | 0.813 |
| High adherence to Med-Diet | 0.55 (0.32–0.92) | 0.023 | 0.56 (0.33–0.97) | 0.038 |
| B | S.E. | Beta | p | |
|---|---|---|---|---|
| Age | −0.01 | 0.01 | −0.05 | 0.273 |
| Female sex | 0.12 | 0.22 | 0.02 | 0.593 |
| Total-c/HDL-c | −0.15 | 0.10 | −0.08 | 0.133 |
| Glycaemia | 0.00 | 0.00 | 0.03 | 0.469 |
| BMI | 0.03 | 0.02 | 0.05 | 0.214 |
| Med-Diet score | −0.19 | 0.06 | −0.12 | 0.002 |
| Triglycerides * | −0.14 | 0.64 | −0.01 | 0.821 |
| APRI * | 1.22 | 0.46 | 0.10 | 0.008 |
| III Pro-C3 Tertile * | II Pro-C3 Tertile * | |||
|---|---|---|---|---|
| Odds Ratio (95% C.I for OR) | p | Odds Ratio (95% C.I for OR) | p | |
| Age ≥ 65 years | 0.60 (0.37–0.97) | 0.038 | 0.57 (0.35–0.92) | 0.023 |
| Female Sex | 1.23 (0.82–1.84) | 0.307 | 1.44 (0.97–2.15) | 0.073 |
| Metabolic Syndrome | 1.00 (0.67–1.50) | 0.993 | 0.89 (0.59–1.34) | 0.579 |
| Antiplatelet therapy | 0.99 (0.58–1.69) | 0.982 | 0.73 (0.42–1.27) | 0.271 |
| APRI > 0.7 | 1.66 (0.75–3.65) | 0.208 | 0.62 (0.23–1.65) | 0.336 |
| High Adherence to Med-Diet | 0.48 (0.27–0.84) | 0.010 | 0.89 (0.54–1.48) | 0.563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, F.; Cammisotto, V.; Tozzi, G.; Coronati, M.; Bartimoccia, S.; Castellani, V.; Nocella, C.; D’Amico, A.; Angelico, F.; Carnevale, R.; et al. High Compliance to Mediterranean Diet Associates with Lower Platelet Activation and Liver Collagen Deposition in Patients with Nonalcoholic Fatty Liver Disease. Nutrients 2022, 14, 1209. https://doi.org/10.3390/nu14061209
Baratta F, Cammisotto V, Tozzi G, Coronati M, Bartimoccia S, Castellani V, Nocella C, D’Amico A, Angelico F, Carnevale R, et al. High Compliance to Mediterranean Diet Associates with Lower Platelet Activation and Liver Collagen Deposition in Patients with Nonalcoholic Fatty Liver Disease. Nutrients. 2022; 14(6):1209. https://doi.org/10.3390/nu14061209
Chicago/Turabian StyleBaratta, Francesco, Vittoria Cammisotto, Giulia Tozzi, Mattia Coronati, Simona Bartimoccia, Valentina Castellani, Cristina Nocella, Alessandra D’Amico, Francesco Angelico, Roberto Carnevale, and et al. 2022. "High Compliance to Mediterranean Diet Associates with Lower Platelet Activation and Liver Collagen Deposition in Patients with Nonalcoholic Fatty Liver Disease" Nutrients 14, no. 6: 1209. https://doi.org/10.3390/nu14061209
APA StyleBaratta, F., Cammisotto, V., Tozzi, G., Coronati, M., Bartimoccia, S., Castellani, V., Nocella, C., D’Amico, A., Angelico, F., Carnevale, R., Pignatelli, P., & Del Ben, M. (2022). High Compliance to Mediterranean Diet Associates with Lower Platelet Activation and Liver Collagen Deposition in Patients with Nonalcoholic Fatty Liver Disease. Nutrients, 14(6), 1209. https://doi.org/10.3390/nu14061209